A kind of ambroxol hydrochloride solution for inhalation and preparation method thereof
A technology of ambroxol hydrochloride and solution, applied in the field of medicine, can solve the problems of lack of uniform standards for drug concentration and course of treatment, lack of effective evidence for respiratory system safety, inconvenience for clinical use and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
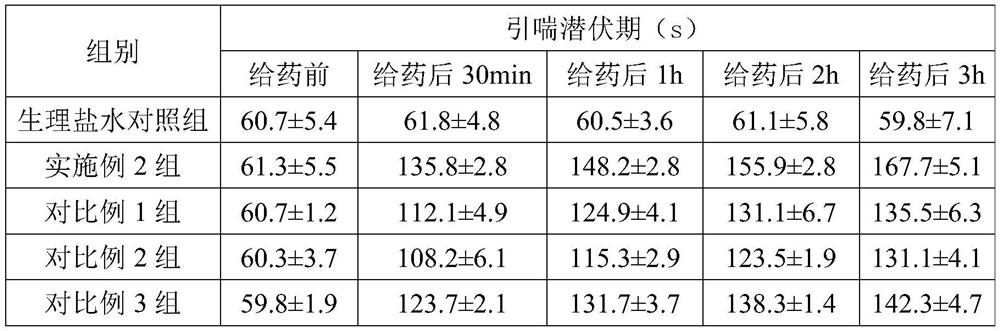
Examples
Embodiment 1
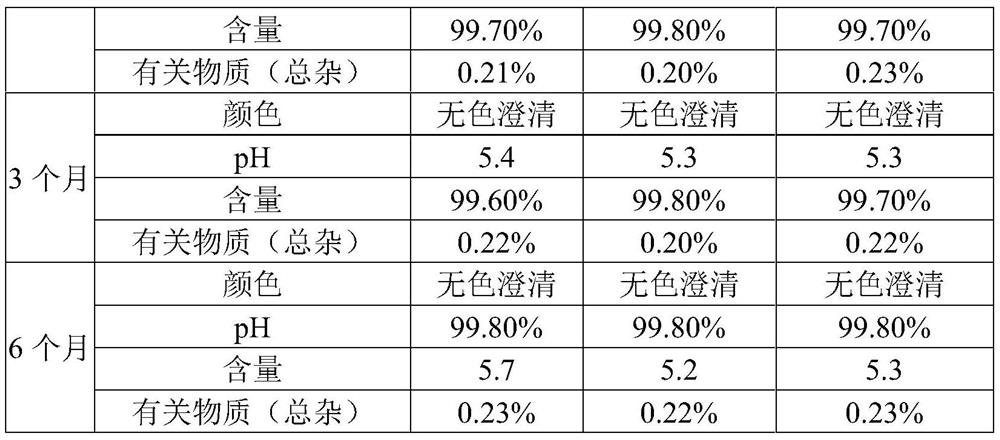
[0034] Embodiment 1 a kind of ambroxol hydrochloride solution for inhalation
[0035] Described inhalation uses ambroxol hydrochloride solution, comprises following composition in every 2000ml water for injection: the ambroxol hydrochloride of 12.0g, the disodium hydrogen phosphate of 1.2g, the citric acid of 1.0g, 10.2g sodium chloride, stable Agent 0.5g and surfactant 0.8g.
[0036] The stabilizer is composed of chitosan and sodium metabisulfite in a weight ratio of 1:7.
[0037] The surfactant is composed of tyloxapol and lecithin in a weight ratio of 6:5.
[0038]The preparation method of described suction ambroxol hydrochloride solution, described preparation method may further comprise the steps:
[0039] S1. Weigh 60% of the water for injection, add disodium hydrogen phosphate, citric acid, ambroxol hydrochloride and surfactant in turn, stir to dissolve, after the dissolution is complete, add sodium chloride and stabilizer, stir Until completely dissolved, mixed solu...
Embodiment 2
[0044] Embodiment 2 a kind of ambroxol hydrochloride solution for inhalation
[0045] Described inhalation uses ambroxol hydrochloride solution, comprises following composition in every 2000ml water for injection: the ambroxol hydrochloride of 15.0g, the disodium hydrogen phosphate of 2.88g, the citric acid of 2.0g, 14.4g sodium chloride, stable Agent 1.0g and surfactant 2.0g.
[0046] The stabilizer is composed of chitosan and sodium metabisulfite in a weight ratio of 2:5.
[0047] The surfactant is composed of tyloxapol and lecithin in a weight ratio of 10:3.
[0048] The preparation method of described suction ambroxol hydrochloride solution, described preparation method may further comprise the steps:
[0049] S1. Weigh 60% of the water for injection, add disodium hydrogen phosphate, citric acid, ambroxol hydrochloride and surfactant in turn, stir to dissolve, after the dissolution is complete, add sodium chloride and stabilizer, stir Until completely dissolved, mixed s...
Embodiment 3
[0054] Embodiment 3 a kind of ambroxol hydrochloride solution for inhalation
[0055] Described inhalation uses ambroxol hydrochloride solution, comprises following composition in every 2000ml water for injection: the ambroxol hydrochloride of 18.0g, the disodium hydrogen phosphate of 4.0g, the citric acid of 3.6g, 16.5g sodium chloride, stable Agent 1.2g and surfactant 2.6g.
[0056] The stabilizer is composed of chitosan and sodium metabisulfite in a weight ratio of 3:4.
[0057] The surfactant is composed of tyloxapol and lecithin in a weight ratio of 11:1.
[0058] The preparation method of described suction ambroxol hydrochloride solution, described preparation method may further comprise the steps:
[0059] S1. Weigh 60% of the water for injection, add disodium hydrogen phosphate, citric acid, ambroxol hydrochloride and surfactant in turn, stir to dissolve, after the dissolution is complete, add sodium chloride and stabilizer, stir Until completely dissolved, mixed so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| micropore | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com