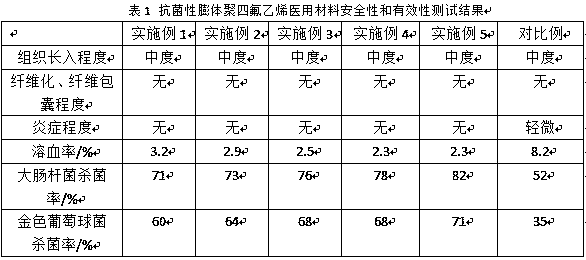
Antibacterial varicosity polytetrafluoroethylene face implant material and preparation technology thereof
A technology of polytetrafluoroethylene and implant materials, which is applied in the direction of prosthesis, coating, medical science, etc., to achieve the effect of simple and efficient process, good bacteriostatic effect and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
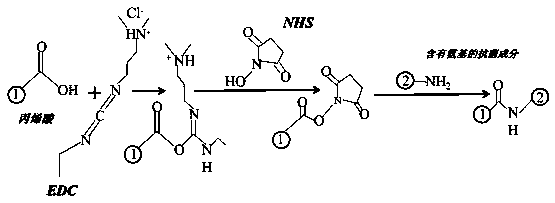
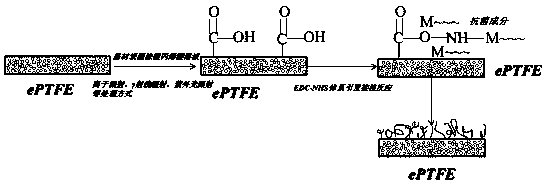
[0031]In this example, atmospheric pressure plasma sputtering is used to activate the grafted acrylic acid on the surface of the expanded polytetrafluoroethylene material, the carboxyl functional group is introduced through the acrylic acid, and then the polymer antibacterial agent chitosan is grafted through the reaction of the carboxyl group and the amino group to endow the material with antibacterial properties , the specific implementation is as follows:
[0032] (1) Cut the medical-grade expanded polytetrafluoroethylene material with an average pore size of 30 μm and a porosity of 50% into a sample with a size of 10×15×2mm, and uniformly coat the surface with a volume fraction of 4% and contain 5mmol / The acrylic acid solution of L ferrous sulfate inhibitor;
[0033] (2) The expanded polytetrafluoroethylene sample coated with acrylic acid solution in step (1) was subjected to atmospheric pressure plasma surface treatment for 4 minutes under the conditions of discharge pow...
Embodiment 2
[0037] In this example, atmospheric pressure plasma sputtering is used to activate the grafted acrylic acid on the surface of the expanded polytetrafluoroethylene material, the carboxyl functional group is introduced through the acrylic acid, and then the polymer antibacterial agent chitosan is grafted through the reaction of the carboxyl group and the amino group to endow the material with antibacterial properties , the specific implementation is as follows:
[0038] (1) Cut the medical-grade expanded polytetrafluoroethylene material with an average pore size of 30 μm and a porosity of 50% into a sample with a size of 10×15×2mm, and uniformly coat the surface with a volume fraction of 6% and contain 10mmol / The acrylic acid solution of L ferrous sulfate inhibitor;
[0039] (2) The expanded polytetrafluoroethylene sample coated with acrylic acid solution in step (1) was subjected to atmospheric pressure plasma surface treatment for 6 minutes under the conditions of discharge p...
Embodiment 3
[0043] In this example, atmospheric pressure plasma sputtering is used to activate the grafted acrylic acid on the surface of the expanded polytetrafluoroethylene material, the carboxyl functional group is introduced through the acrylic acid, and then the polymer antibacterial agent chitosan is grafted through the reaction of the carboxyl group and the amino group to endow the material with antibacterial properties , the specific implementation is as follows:
[0044] (1) Cut the medical-grade expanded polytetrafluoroethylene material with an average pore size of 30 μm and a porosity of 50% into a sample with a size of 10×15×2 mm, and uniformly coat the surface with a volume fraction of 8% and contain 15mmol / The acrylic acid solution of L ferrous sulfate inhibitor;
[0045] (2) The expanded polytetrafluoroethylene sample coated with acrylic acid solution in step (1) was subjected to atmospheric pressure plasma surface treatment for 8 minutes under the conditions of discharge ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture size | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com