Amphiphilic dinaphthalene derivative containing double hydroxyls as well as preparation method and application of amphiphilic dinaphthalene derivative containing double hydroxyls in distinguishing chiral diaminocyclohexane gas
A technology of cyclohexanediamine and derivatives is applied in the field of supramolecular fluorescence sensing thin film materials, and achieves the effects of high yield, simple preparation method and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1. Preparation of the compound of formula I
[0040] Dissolve 1.51g (8.07mmol) 6-hydroxy-2-naphthylboronic acid and 1.17g (11.25mmol) 2,2-dimethyl-1,3-propanediol in 50mL purified dichloromethane at 35℃ The reaction was stirred for 4 hours. After the reaction was completed, the dichloromethane was evaporated under reduced pressure to obtain a crude product. The crude product was purified by column chromatography with a mixed solvent of 1:1 volume ratio of ethyl acetate and petroleum ether as the mobile phase and silica gel as the stationary phase. After vacuum drying, 1.72 g of a white solid was obtained, namely the compound of formula I. The yield was 83.5% . The reaction equation is as follows:
[0041]
[0042] The hydrogen nuclear magnetic spectrum characterization results of the obtained compound of formula I are as follows: 1 H NMR(600MHz, CDCl 3 ,Me 4 Si)δ H : 8.28 (1H), 7.79 (2H), 7.65 (1H), 7.13 (1H), 7.08 (1H), 3.87 (4H),
[0043] 1.05 (6H).
[0044] 2. Preparation...
Embodiment 2
[0057] The application of the dihydroxy-containing amphiphilic binaphthyl derivative in the discrimination of chiral cyclohexanediamine gas in Example 1 is as follows:
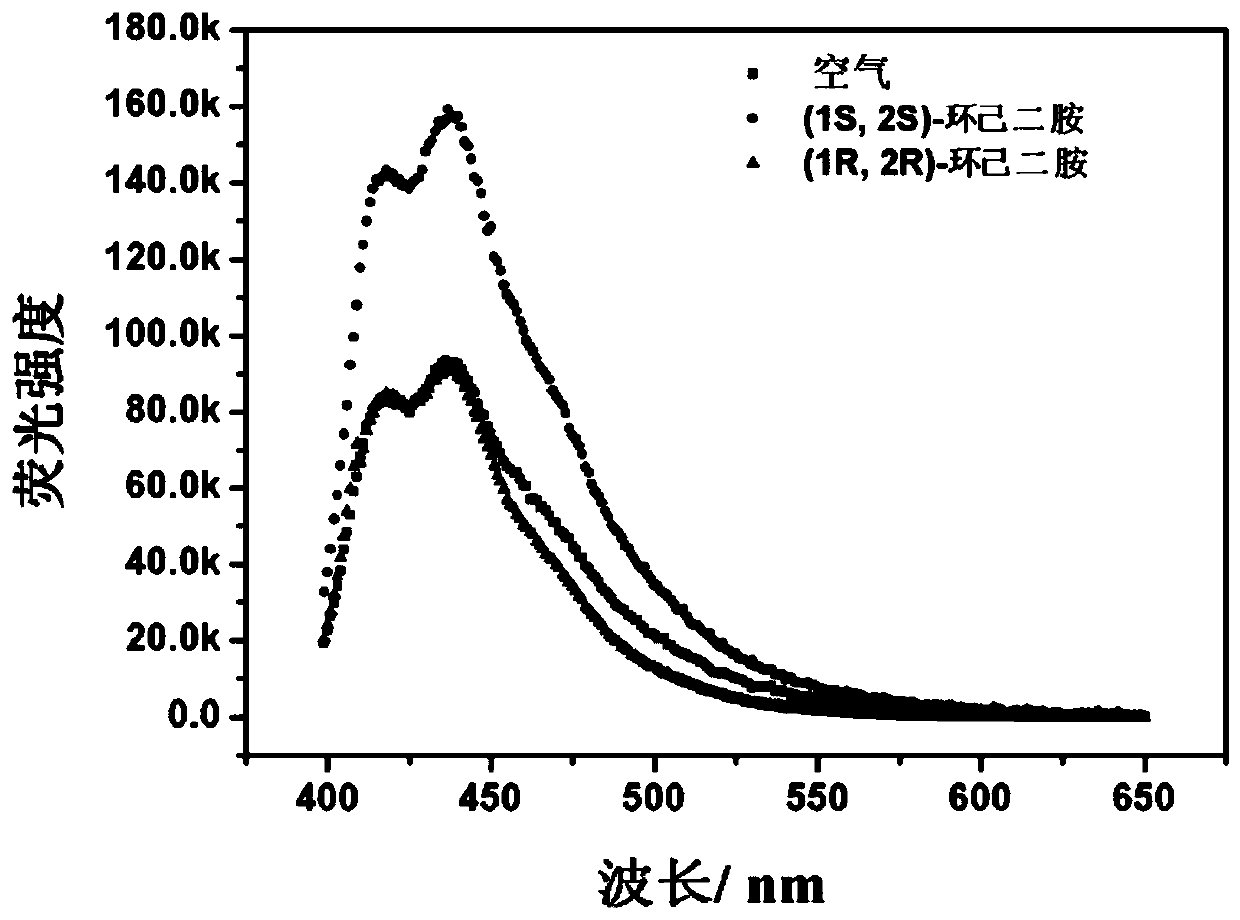
[0058] Dissolve the dihydroxy-containing amphiphilic binaphthyl derivative in PEG 200 to prepare a 60μmol / L dihydroxy-containing amphiphilic binaphthyl derivative stock solution; store the dihydroxy-containing amphiphilic binaphthyl derivative The liquid is in contact with the gold substrate with hydrophilic and hydrophobic microdomains, and an ordered pattern with a droplet diameter of 50μm is formed in the hydrophilic mercaptoundecanoic acid monolayer area, and a microarray is prepared into a gas-liquid interface monolayer fluorescence transmission. Sense film (see figure 1 with figure 2 ).
[0059] Place the above-mentioned fluorescent sensing film in the air without chiral cyclohexanediamine, use FLS980 single photon counting time-resolved fluorescence spectrometer to measure the fluorescence emission spectrum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com