(R)-TAPP-BINOL-COF polymer as well as preparation method and application thereof
A TAPP-BINOL-COF and -BINOL-CHO technology is applied in the direction of organic chemical methods, chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, etc., which can solve the problem of poor chiral selectivity and low yield. High production cost of clopidogrel and increase of wastes, etc., to achieve the effect of easy separation, low price, high yield and stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] Preferably, the preparation method includes: adding Cu-TAPP and (R)-BINOL-CHO to a mixed solution of dilute acetic acid, mesitylene and 1,4-dioxane for reaction.
[0052] Preferably, the reaction temperature is 110-130°C.
[0053] Preferably, the reaction time is 2.5-3.5d.
[0054] Preferably, the molar mass ratio of Cu-TAPP to (R)-BINOL-CHO is 0.8-1.2:1.8-2.2.
[0055] Preferably, the volume ratio of the dilute acetic acid, mesitylene and 1,4-dioxane is 2-4:4-6:14-17.
[0056] Preferably, the preparation method further includes: after the reaction is completed, collecting the solid part and washing with ethanol to obtain a purple-black powder, that is, the polymer (R)-TAPP-BINOL-COF.
[0057] The third aspect of the present disclosure provides the use of the polymer described in the first aspect as a catalyst.
[0058] Preferably, the catalyst is a clopidogrel intermediate catalyst; further, as (S)-2-(2-chlorophenyl)-2-(6,7-dihydrothieno[3,2-c ]pyridin-5 (4H)-yl) a...
Embodiment 1
[0067] Embodiment 1: the synthesis of (R)-BINOL-CHO
[0068] Put R-1,1'-bi-2-naphthol (10.0mmol, 2.86g) in a 100ml three-necked flask, add dichloromethane (20ml), cool to 0°C, and slowly add liquid bromine ( 25.2mmol, 4.00 g), after the dropwise addition, the reaction solution was light orange. After continuing the reaction at 0°C for 24 h, add Na 2 S 2 o 3 (7.4 mmol, 1.20g) of aqueous solution, continue to stir for 2h. After the reaction was completed, the color of the reaction solution changed from orange to light yellow. Filter the reaction solution, transfer the filtrate to a separatory funnel, wash the organic phase three times with saturated NaCl solution, combine the organic phases, and add an appropriate amount of NaCl 2 SO 4 After drying, the solvent was removed by rotary evaporation to obtain (R)-6,6'-dibromo-1,1'-bi-2-naphthol as a light yellow solid product.
[0069] Weigh (R)-6,6'-dibromo-1,1'-bi-2-naphthol (10.0mmol, 4.44g) into a 100ml flask, add acetone ...
Embodiment 2
[0073] Example 2: Synthesis of (R)-TAPP-BINOL-COF
[0074] Cu-TAPP (0.05mmol, 36.60mg), (R)-BINOL-CHO (0.10mmol, 61.60 mg), dilute acetic acid (6M, 0.30ml) and 1,4-dioxane / mesitylene (2ml , volume ratio = 3:1) solution into the pressure tube. After freezing with liquid nitrogen, vacuumize, and then thaw, the above operation was repeated three times, and then placed in a 120°C constant temperature oven to react for 3 days. After cooling to room temperature, it was collected by centrifugation, washed repeatedly with ethanol, and vacuum-dried to obtain a purple-black Cu-COF powder with a yield of 82%.
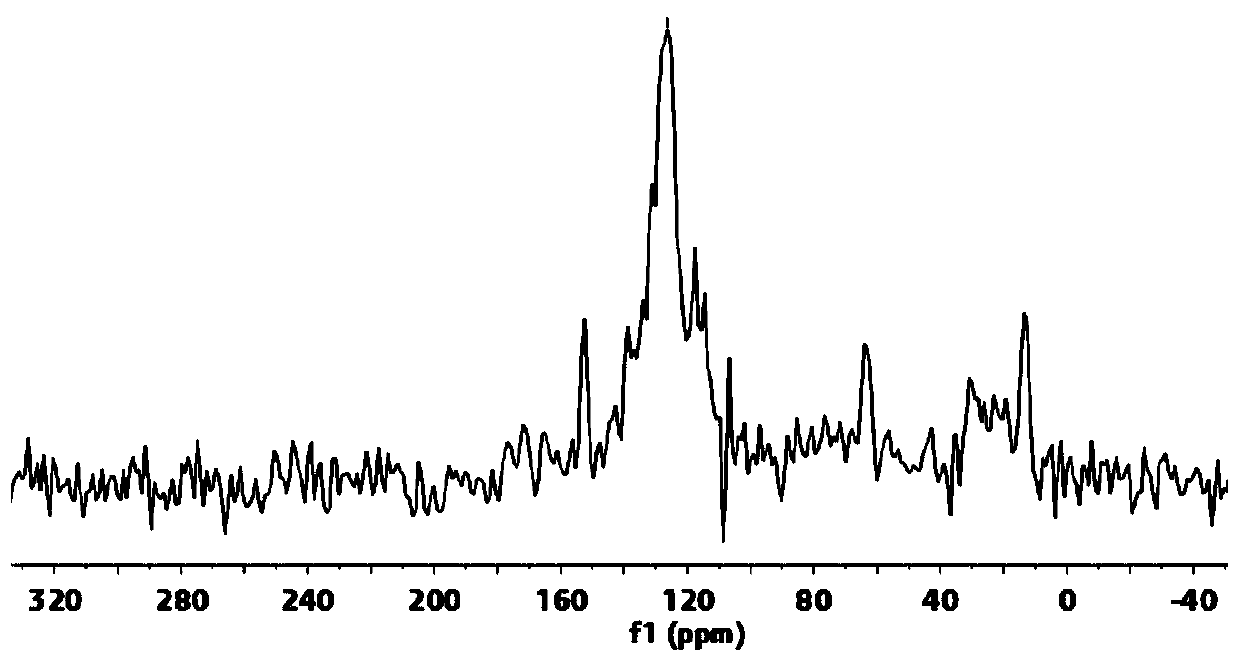
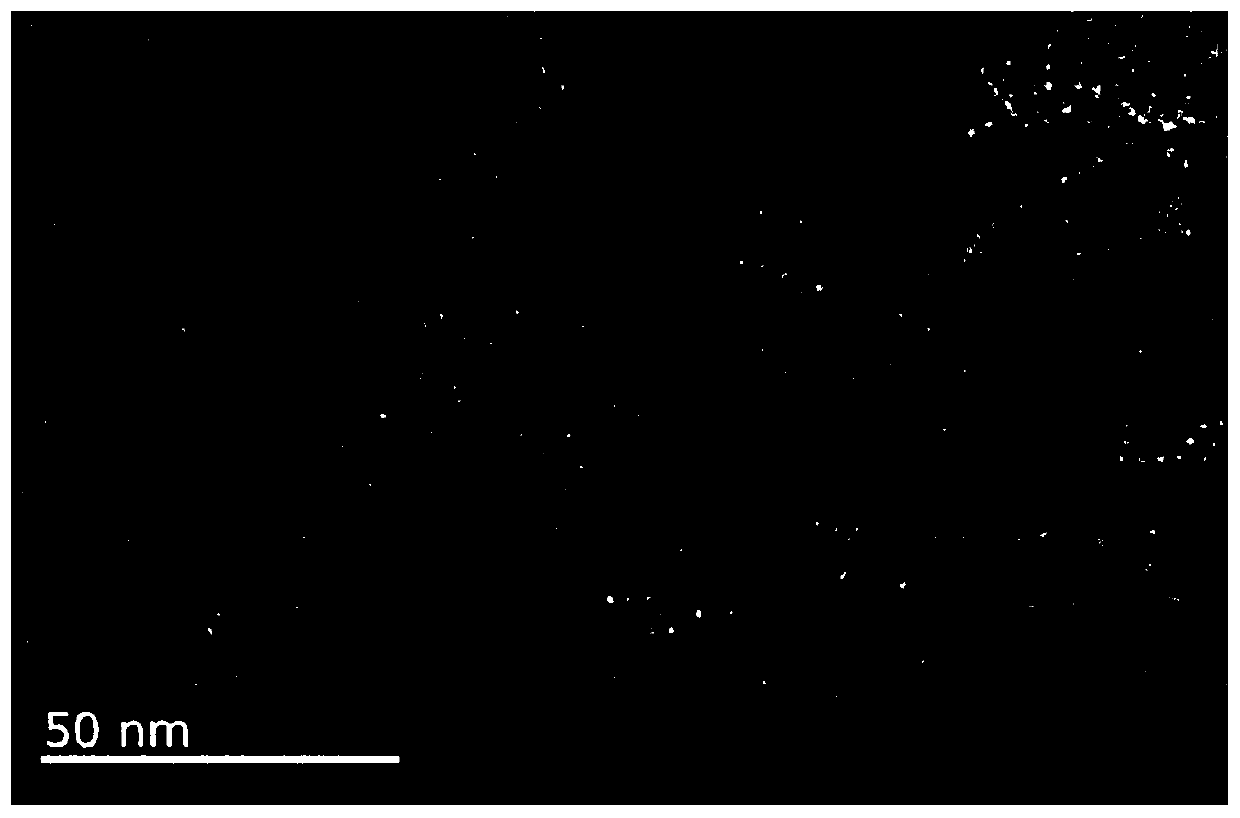
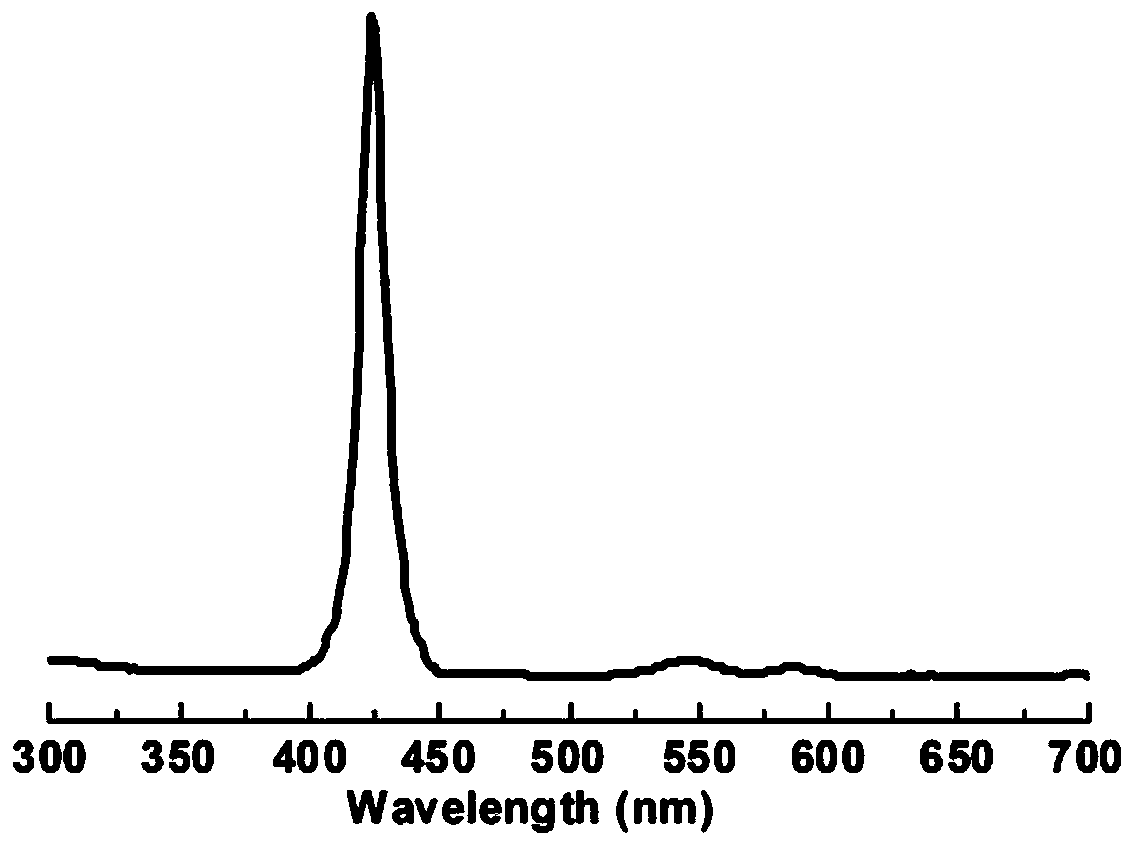
[0075] In this embodiment, solid NMR, PXRD, N 2 The compound was characterized by adsorption and TGA, and the results are shown in figure 1 , 5 , 6 and 7. It can be seen that the polymer has a hollow structure. The ultraviolet absorption of (R)-TAPP-BINOL-COF prepared by the above method in acetonitrile solution ( image 3 ) shows that it has the maximum absorption at 420nm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com