Gold-copper-boron porous network structure electrocatalyst and production method thereof
A network structure and electrocatalyst technology, applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, etc., can solve problems such as time-consuming and complicated methods, and achieve outstanding results Activity and stability, simple preparation method, performance-enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] A kind of preparation method of gold-copper-boron porous network structure electrocatalyst, described method comprises the steps:
[0053] 1) Prepare copper chloride and chloroauric acid N,N-dimethylformamide solutions with a concentration of 20mM respectively;
[0054] 2) Mix 2mL of copper chloride and 2mL of chloroauric acid N,N-dimethylformamide solution, and then add 20mL of 20mM freshly prepared sodium borohydride N,N-dimethylformamide under vigorous stirring at room temperature Amide solution;
[0055] 3) After reacting for 5 minutes, wash, centrifuge, and dry to obtain the gold-copper-boron porous network electrocatalyst.
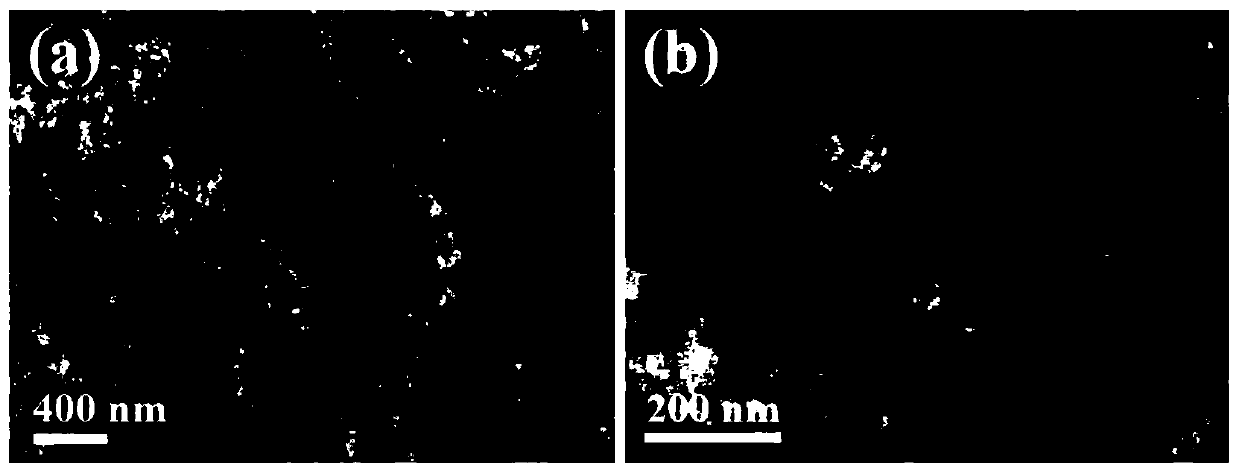
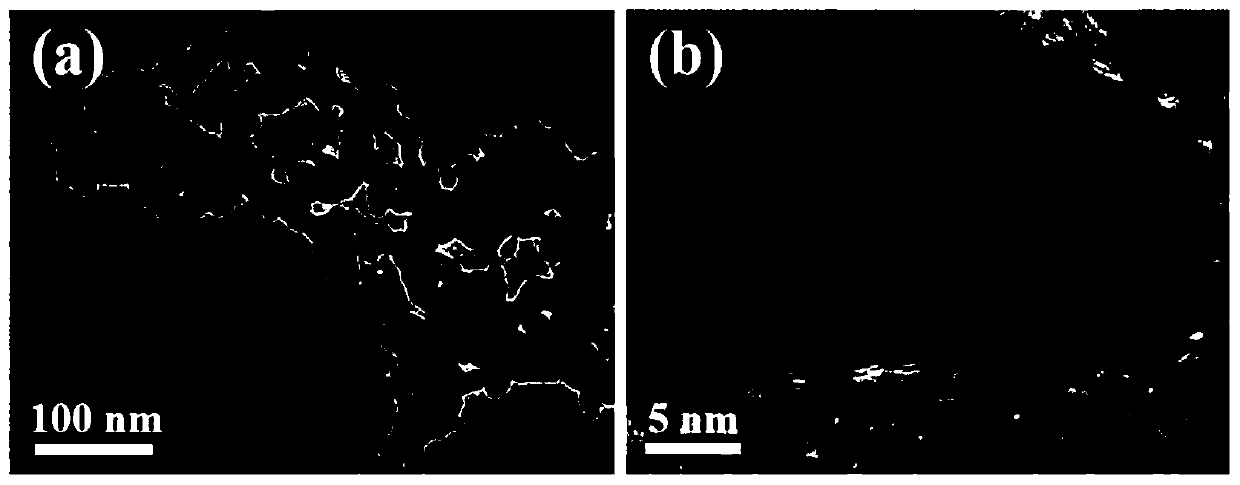
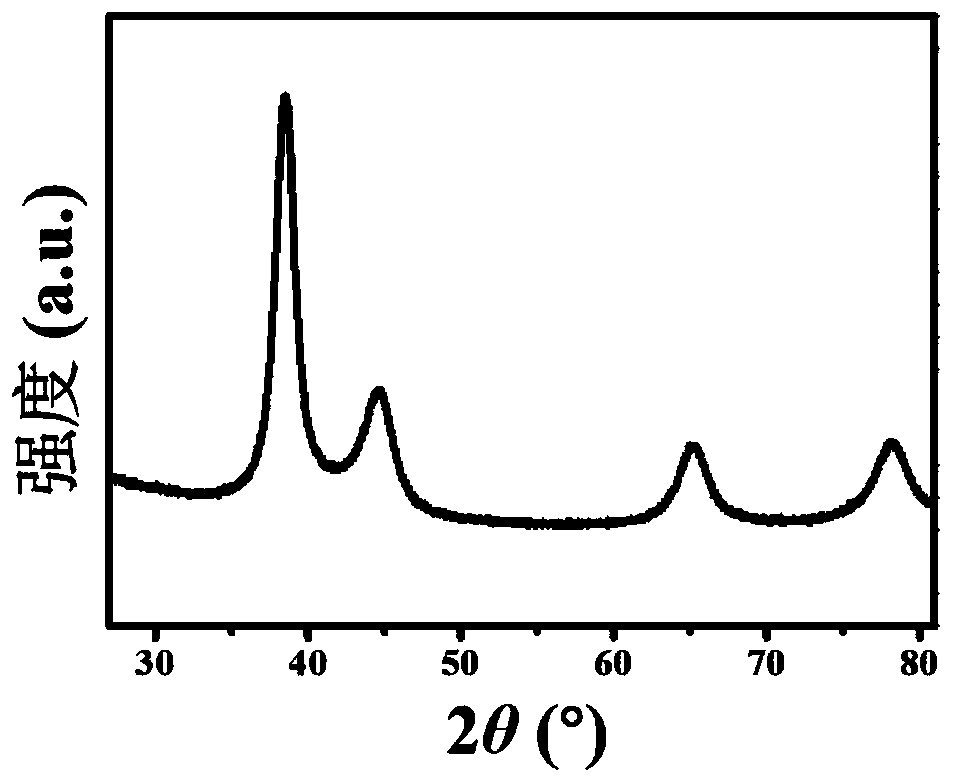
[0056] The SEM picture of the obtained gold-copper-boron porous network structure can be found in figure 1 . The TEM picture of the obtained gold-copper-boron porous network structure can be found in figure 2 . The XRD pattern of the obtained gold-copper-boron porous network structure can be found in image 3 . The XPS figure of the go...
Embodiment 2
[0059] A kind of preparation method of gold-copper-boron porous network structure electrocatalyst, described method comprises the steps:
[0060] 1) Prepare copper chloride and chloroauric acid N,N-dimethylformamide solutions with a concentration of 1mM respectively;
[0061] 2) Mix 2 mL of copper chloride and 2 mL of chloroauric acid N, N-dimethylformamide solution, and then add 20 mL of freshly prepared sodium borohydride N, N-dimethylformamide with a concentration of 1 mM under vigorous stirring at room temperature. Formamide solution;
[0062] 3) After reacting for 1 min, wash, centrifuge, and dry to obtain the gold-copper-boron nanoparticle electrocatalyst.
[0063] Since the concentration of chloroauric acid and copper chloride is very low in this process, and the reaction temperature is short, it is difficult to centrifuge out of the solution, so it is difficult to synthesize a gold-copper-boron catalyst with a porous network structure. Obtain the SEM figure of gold-c...
Embodiment 3
[0065] A kind of preparation method of gold-copper-boron porous network structure electrocatalyst, described method comprises the steps:
[0066] 1) Prepare copper chloride and chloroauric acid N,N-dimethylformamide solutions with a concentration of 40mM respectively;
[0067] 2) Mix 2mL of copper chloride and 2mL of chloroauric acid N,N-dimethylformamide solution, and then add 20mL of 40mM freshly prepared sodium borohydride N,N-dimethylformamide under vigorous stirring at room temperature Amide solution;
[0068] 3) After reacting for 60 minutes, wash, centrifuge, and dry to obtain the gold-copper-boron nanoparticle electrocatalyst.
[0069] Because in this process, the concentration of chloroauric acid and copper chloride N,N-dimethylformamide is relatively large, and the reaction time increases, which will cause more copper to be reduced, resulting in blocky agglomerates, which are difficult to use Application of electrochemical synthesis of ammonia.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com