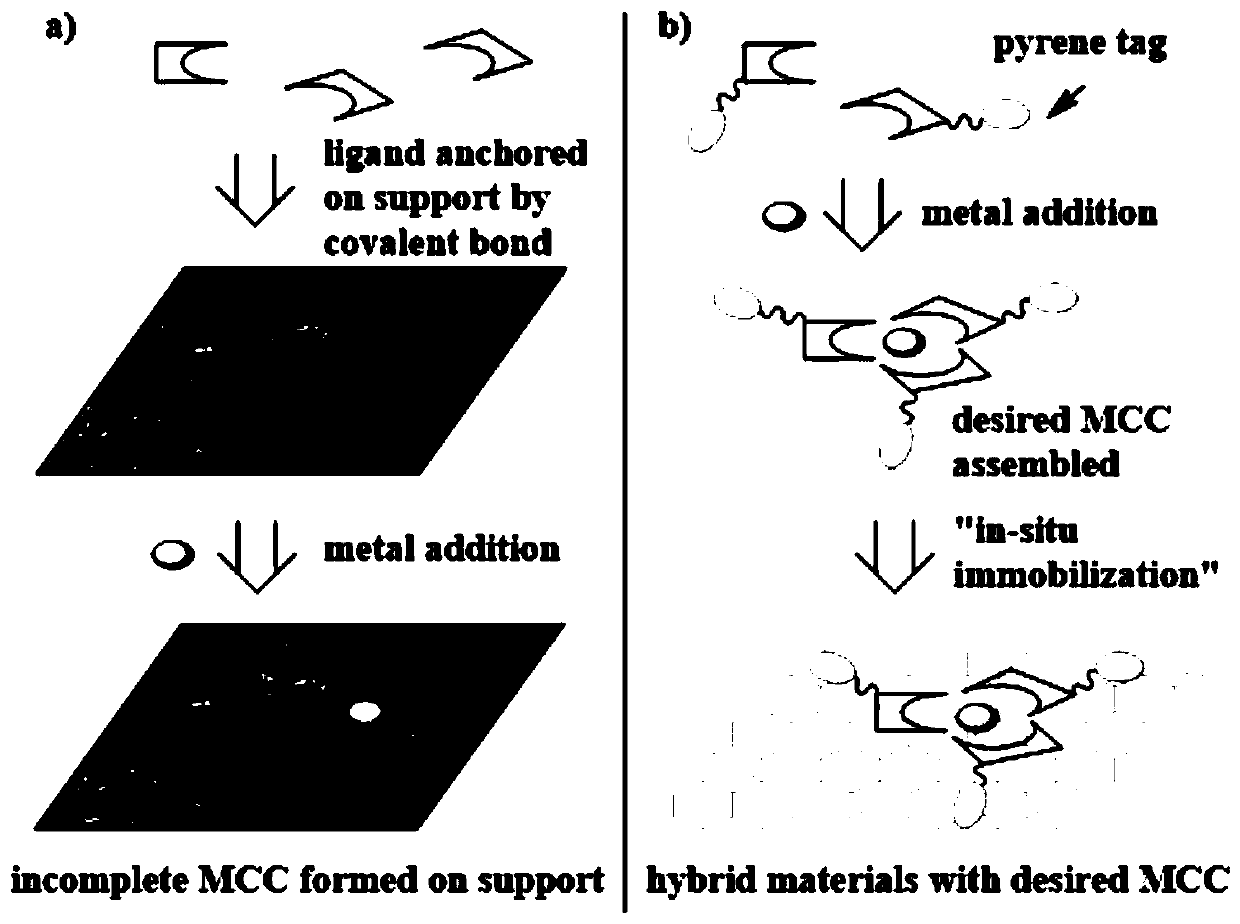
Graphene adsorption multi-component chiral catalyst and application thereof in asymmetric hydrogenation
A chiral catalyst, graphene technology, used in catalytic reactions, asymmetric synthesis, physical/chemical process catalysts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Synthesis of hybrid material 2a adsorbed on graphene
[0029] Preparation of Compound S3
[0030] 224mg 1-pyrenebutanoic acid S1 (0.78mmol), 300mg R-[1,1'-naphthalene]-2,2'-dimethoxy-6-butanol S2 (0.78mmol), 320mg DCC and 8mL di Chloromethane was mixed, then 190 mg DMAP (1.55 mmol) was added and stirred overnight at room temperature. The reaction mixture was quenched with celite, stirred for 1 h, filtered and concentrated in vacuo to obtain a light yellow oily crude product, which was purified by silica gel column chromatography (ethyl acetate / petroleum ether=1:10) to obtain 480 mg of S3 with a yield of 94%. Pale yellow solid. mp:72℃; [ɑ]=+30.6°(20℃, c=0.01g / ml, DCM). 1 H NMR (400MHz, CDCl 3 )δ8.32(d,J=9.1Hz,1H),8.19-8.14(m,2H),8.13-8.06(m,2H),8.07-7.95(m,4H),7.94-7.82(m,3H) ,7.65(s, 1H),7.49-7.41(m,2H),7.38-7.31(m,1H),7.25-7.22(m,1H),7.19-- 7.15(m,1H),7.08(s,2H ),4.23-4.14(m,2H),3.77(s,6H),3.40(t,J=7.4Hz,2H),2.76(t,J=7.4Hz,2H),2.49-2.45(m,2H) ,2.25-2.2...
Embodiment 2
[0046] Example 2 Hybrid material 2a hydrogenation catalytic reaction adsorbed on graphene
[0047] The hybrid material 2a@graphene adsorbed on graphene catalyzes the asymmetric hydrogenation of dehydroamino acids 6a-h, and the results are as follows:
[0048]
[0049] The specific operation is as follows: under argon protection, mix [Rh (COD) 2 ] BF 4 (2.0 mg, 0.005 mmol) and ligand 2 (7.0 mg, 0.01 mmol) were prepared in situ to obtain catalyst 2a. Graphene (20mg) was added in the above solution and stirred for 30 minutes to obtain the hybrid material 2a@graphene adsorbed on the graphene. The hybrid material 2a@graphene (0.01mmol, 10mM, based on (Monophos) 2 / Rh unit), substrate 6 (1 mmol, 1.0 M) in anhydrous ethyl acetate (5.0 mL). The test tube was placed in a stainless steel autoclave and sealed, replaced with hydrogen 3 times, the final hydrogen pressure was adjusted to 20 atm and stirring was started. At the end of the reaction (1.5 h), hydrogen was released and t...
Embodiment 3
[0058] Example 3 2a@graphene reuse
[0059] 2a@graphene adsorbed on graphene is filtered at the end of the reaction in Example 2, and continues to be used in the asymmetric hydrogenation of phenyl β-dehydroamino acid ester 6a, using the same reaction conditions as in Example 2, the number of cycles and conversion rate are the same as those for Mapping selectivity relationship ( Figure 5 ).
[0060] The results show that under the action of the same catalyst, the hydrogenation reaction has nearly quantitative conversion and stable enantioselectivity (96-92% ee), and has been recycled at least 13 times. However, after the catalyst was cycled 7 times, it was necessary to extend the reaction time from 1.5 hours to 10 hours to ensure complete conversion of the substrate, indicating that the hybrid material 2a@graphene adsorbed by the catalyst on graphene was reactive during the continuous hydrogenation process. Gradually decreases.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com