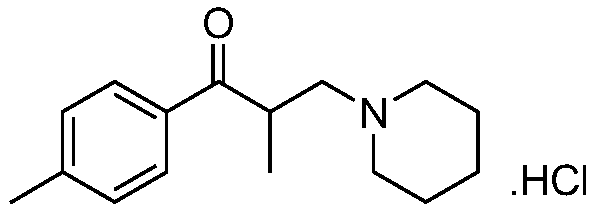
Method for preparing high-purity tolperisone hydrochloride
A technology of tolapazone hydrochloride and preparation process, applied in the field of medical technology, can solve the problems of complicated operation and high cost, and achieve the effects of high yield, good quality and shortening reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] With reference to the European Pharmacopoeia method, HPLC is used to test tolperone hydrochloride;
[0047] Step (1): Add 99.6g of toluene and 86.5g of anhydrous aluminum trichloride to the 250ml reaction bottle in sequence, and cool down to below 0°C;
[0048] Step (2): Slowly add 50g of propionyl chloride dropwise, and control the internal temperature below 0°C, then raise the temperature to 30-40°C, and react for 3 hours;
[0049] Step (3): After the reaction, slowly pour the reaction solution into 500 g of ice-water mixture, and control the internal temperature to be less than or equal to 15°C to quench and separate the liquids, concentrate the toluene phase and spin dry to obtain high-purity p-methylpropiophenone;
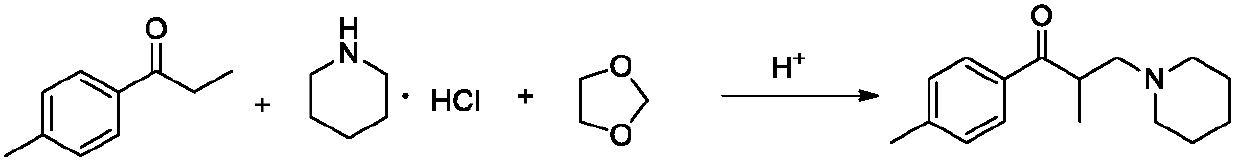
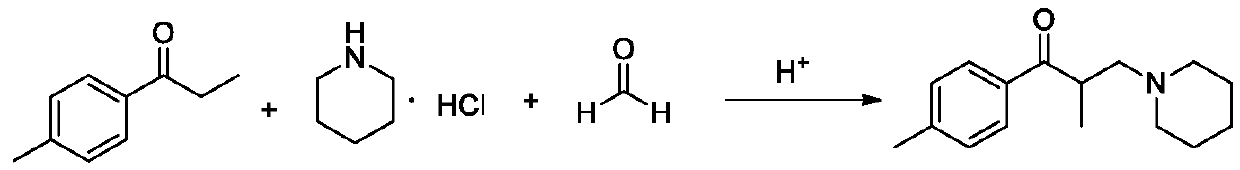
[0050] Step (4): Add 70g of p-methylpropiophenone, 46g of piperidine hydrochloride, 18.4g of paraformaldehyde and 46g of isopropanol into a 1000ml three-necked reaction flask, stir, heat up to an internal temperature of 90-100°C, and react 5 Hour;
[...
Embodiment 2
[0054] Compared with Example 1, the only difference is that in step (2), when slowly dripping 50g propionyl chloride, the internal temperature is controlled at 0-10°C; the product purity and impurity control are similar to Example 1, but the mass ratio of Example 1 The difference of 1; Owing to raising the temperature of dripping propionyl chloride, cause Friedel-Crafts acylation reaction by-product to increase, thereby influence product quality.
Embodiment 3
[0056] Compared with Example 1, the only difference is that in step (6), when the temperature is lowered to 25-35° C., there is no heat preservation reaction for 2 hours, and the total yield of refined products is only 47%. The product purity and impurity control are similar to Example 1. But the yield is low; because the heat preservation reaction was not continued for 2 hours at 25-35° C., the yield was low, which affected the product yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com