EGFR (epidermal growth factor receptor)-specific chimeric antigen receptor and application thereof
A chimeric antigen receptor and specific technology, applied in the fields of molecular biology and immunology, can solve the problems of limited anti-tumor effect, increased expression level, strong depletion, etc., and achieve strong tumor-specific antigen activation, Enhanced durability, good applicability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] This embodiment is the packaging and preparation method of EGFR-CAR recombinant lentiviral vector, the EGFR-CRA includes EGFR-Fc-CAR (second generation) as shown in SEQ ID NO.09, EGFR as shown in SEQ ID NO.10 -Fc-CAR (third generation), EGFR-Hinge-CAR (second generation) as shown in SEQ ID NO.11 and EGFR-Hinge-CAR (third generation) as shown in SEQ ID NO.12, the nucleotide sequences are as follows Shown in SEQ ID NO.13-16.
Embodiment 2
[0082] This example is a detection method for chimeric antigen receptor expression on 293T.
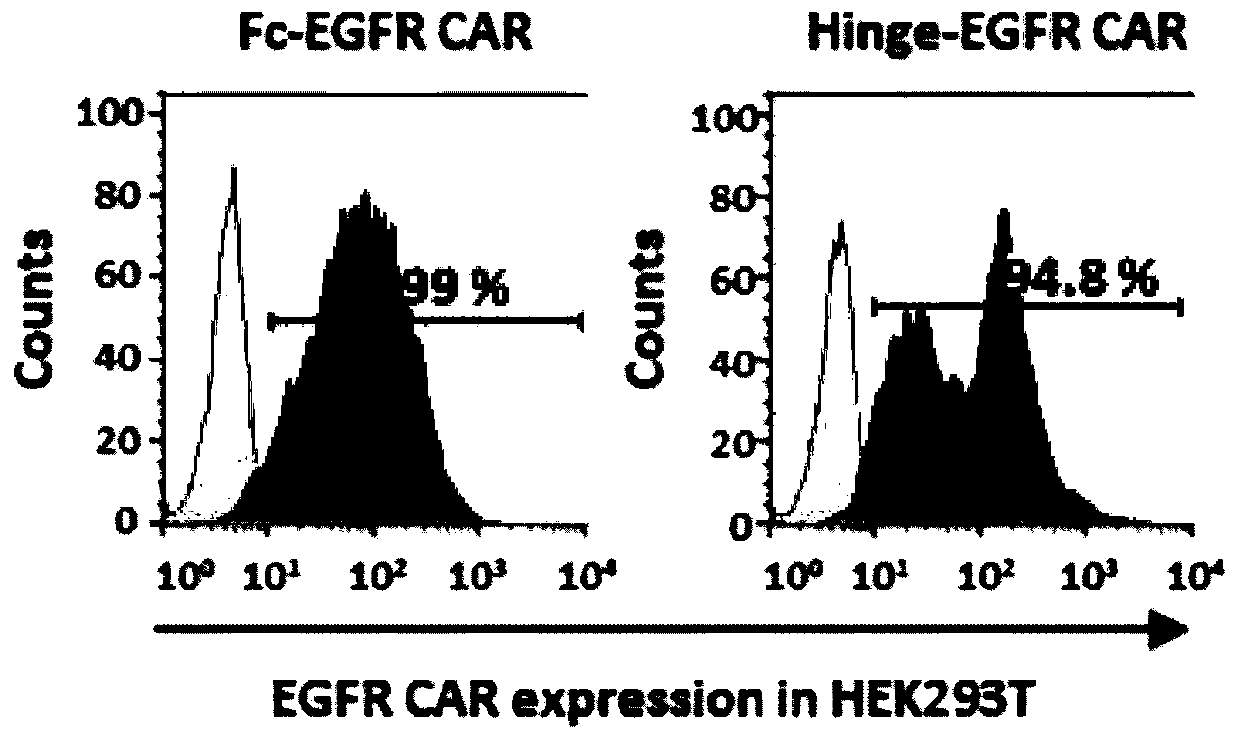
[0083] (1) Flow cytometry detection of chimeric antigen receptor expression on the surface of 293T cells
[0084] Collect the 293T cells transfected for 60 h in Example 1, centrifuge at 1000 rpm for 5 min to remove the supernatant, wash twice with PBS, and then wash with 1×10 6 cells / mL were added to PBS to suspend cells, and the corresponding antibody was added to protect from light at 4°C for 40 minutes, then washed with PBS, resuspended, and finally detected by flow cytometry. Wherein Fc-EGFR-CAR is detected by anti-human IgG1 Fc antibody; Hinge-EGFR-CAR is detected by anti-human IgG(Fab')2 antibody.
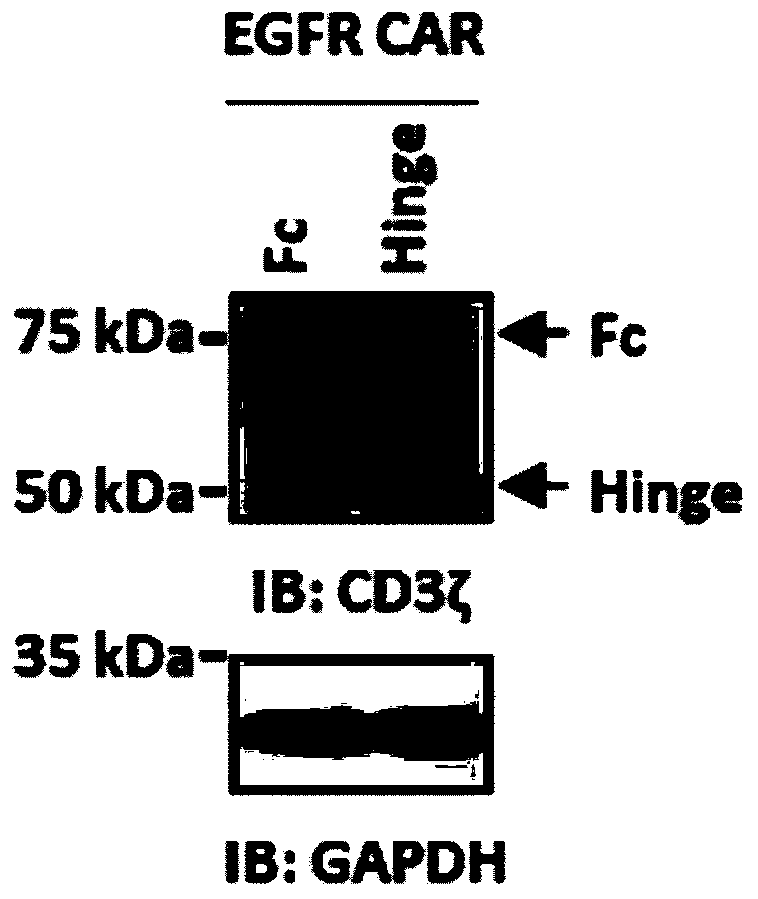
[0085] (2) Western blot verification of chimeric antigen receptor expression in 293T cells
[0086] The 293T cells transfected for 60 hours in Example 1 were collected, lysed with RIPA lysate, sonicated, centrifuged to obtain the supernatant, measured for concentration and quantifi...
Embodiment 3
[0090] This example is the isolation and culture of human primary T lymphocytes.
[0091] Lymphocytes were separated by human peripheral blood lymphocyte separation medium Ficoll (produced by Tianjin Haoyang Company), and X-VIVO (produced by LONZA Company) medium containing 10% FBS, CD3, CD28 antibodies and cytokines was used as a T cell culture medium. Adjust the cell density to 1 x 10 6 Cells / mL were cultured, and the expressions of T cell surface markers CD3 and CD8 were detected by flow cytometry after stimulation for 72 hours, and the lentivirus prepared in Example 1 was used for infection.
[0092] The result is as Figure 4 As shown, 99% of the isolated and cultured lymphocytes are CD3-positive cells, and 95% are CD8-positive effector cells, indicating that effector T cells that can be used for lentivirus infection to prepare CAR-T have been isolated.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com