Cleaning solution or diluent thereof special for full-automatic chemiluminescence tester and preparation method thereof
An automatic chemistry and cleaning solution technology, which is applied in chemical instruments and methods, chemiluminescence/bioluminescence, and analysis through chemical reactions of materials, etc. It can solve the problems of unsatisfactory anti-corrosion effects, unsuitable cleaning solutions, and increased detection costs, etc. problem, to achieve the effect of good accuracy, low cost and high precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-4
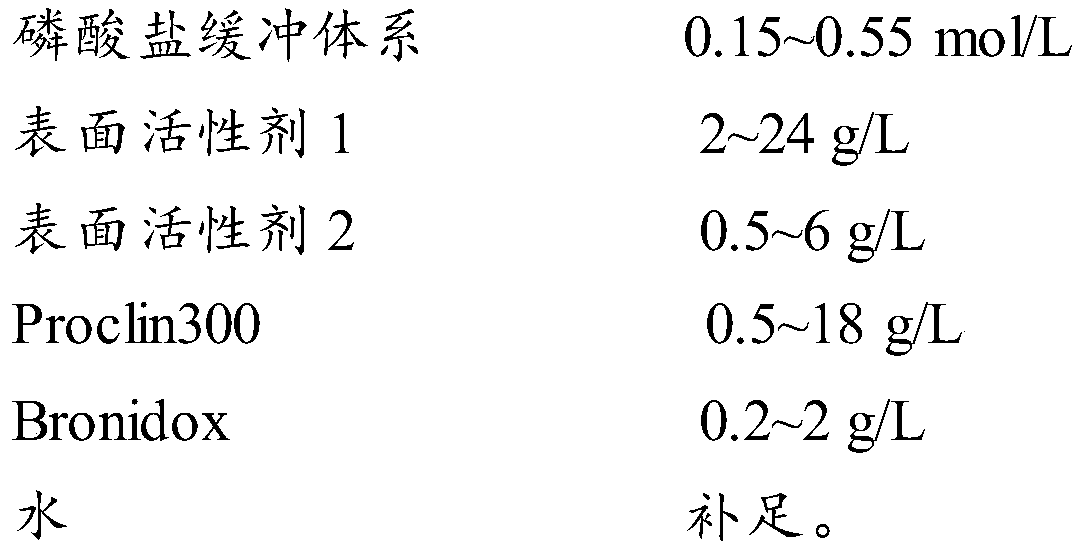
[0056] Table 1 shows the formula ratio of the cleaning solution of Examples 1-4 of the present invention and the multiple of dilution during use.
[0057] Table 1 Example 1-4 formula table and dilution factor during use
[0058]
[0059] Taking the example 1 cleaning solution of preparing 1L as an example, the preparation method is as follows:
[0060] Weigh 35g sodium chloride g, 185g potassium chloride, 53.72g disodium hydrogen phosphate dodecahydrate, 0.13g potassium dihydrogen phosphate, 24g Tween-20, 0.5g sodium laurate, 10g Proclin300, 0.4g Bronidox plus purification After stirring and dissolving in water, dilute to 1L. The pH value of the cleaning solution is 7.2-8.3. Dilute 15 times with purified water when used as a working solution.
[0061] The preparation technical parameter of embodiment 2-4 is the same as embodiment 1.
Embodiment 1
[0074] The deviations of the measured values of the cleaning solution in Example 1 and the original cleaning solution sample are all within 3%, indicating that the cleaning solution of the present invention has a good test accuracy and is consistent with the measurement results of the original cleaning solution.
[0075] 2. Precision experiment
[0076] Abbott i2000SR chemiluminescence immunoassay analyzer is used to measure the original cleaning solution and the cleaning solution of Example 1 respectively, and each item measures the corresponding precision quality control product (repeated 20 times), and the selected items are respectively the surface of hepatitis B virus of sandwich method Antigen (HBsAg) and thyroid-stimulating hormone (TSH), estradiol (E2) in the competition method, herpes simplex virus type 2 IgG antibody (HSV-2 IgG) in the indirect method, rubella virus antibody (IGM) precision in the capture method The measurement results are shown in Table 3.
[007...
Embodiment 2
[0108] The deviations of the measured values of the cleaning solution in Example 2 and the original cleaning solution samples are all within 3%, indicating that the cleaning solution of the present invention has a good test accuracy and is consistent with the measurement results of the original cleaning solution.
[0109] 2. Precision experiment
[0110] Abbott i2000SR chemiluminescence immunoassay analyzer is used to measure the original cleaning solution and the cleaning solution of Example 2 respectively, and each item measures the corresponding precision quality control product (repeated 20 times), and the selected items are respectively the surface of hepatitis B virus of the sandwich method Antigen (HBsAg) and thyroid-stimulating hormone (TSH), estradiol (E2) in the competition method, herpes simplex virus type 2 IgG antibody (HSV-2 IgG) in the indirect method, rubella virus antibody (IGM) precision in the capture method The measurement results are shown in Table 7.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com