A kind of polyimide fiber and preparation method thereof
A technology of polyimide fiber and polyamic acid fiber, applied in the field of fibers, can solve the problems of easy agglomeration, poor heat aging resistance, large specific surface area of nanoparticles, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] The invention provides a kind of preparation method of polyimide fiber, comprises the following steps:
[0054] a) Aromatic dianhydride monomers and diamine monomers are polymerized in a solvent to obtain a polyamic acid spinning stock solution;
[0055] b) spinning the polyamic acid spinning stock solution to obtain polyamic acid fibers;
[0056] c) performing imidization treatment on the polyamic acid fibers to obtain polyimide fibers;
[0057] The diamine monomer comprises monomer A and monomer B;
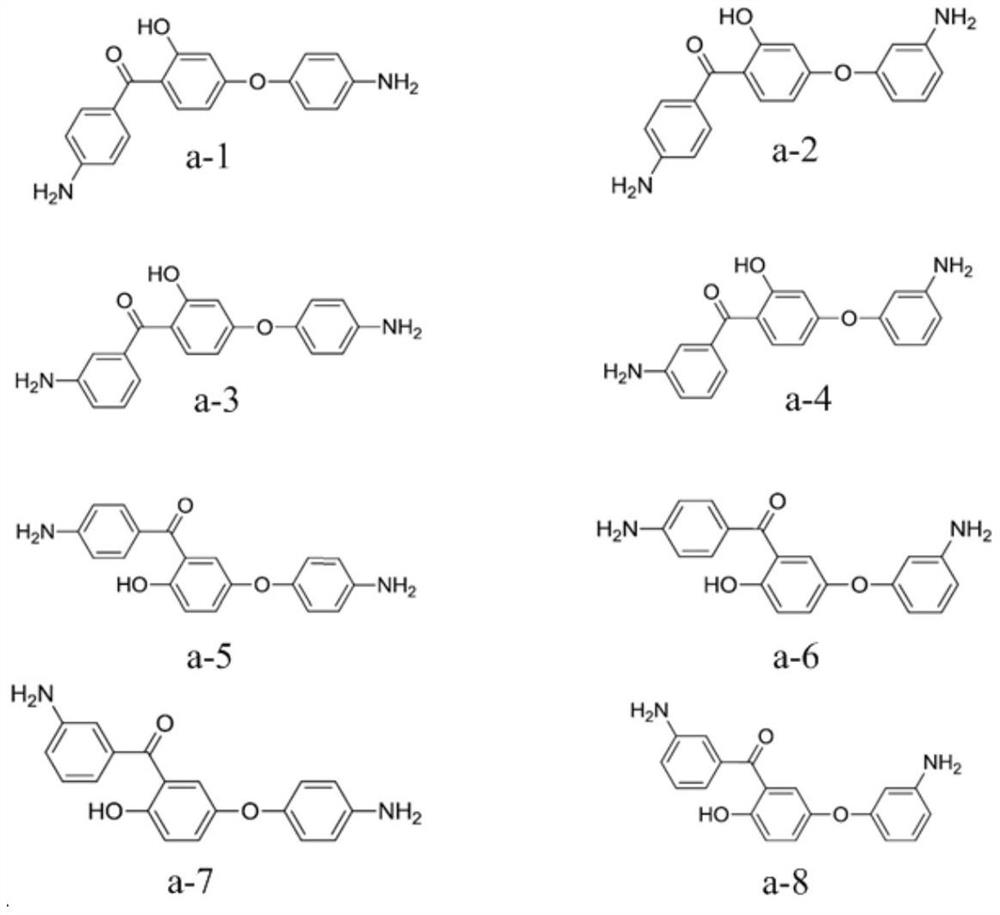
[0058] The monomer A is selected from one or more of the structures shown in formula a-1 to formula a-8:
[0059]
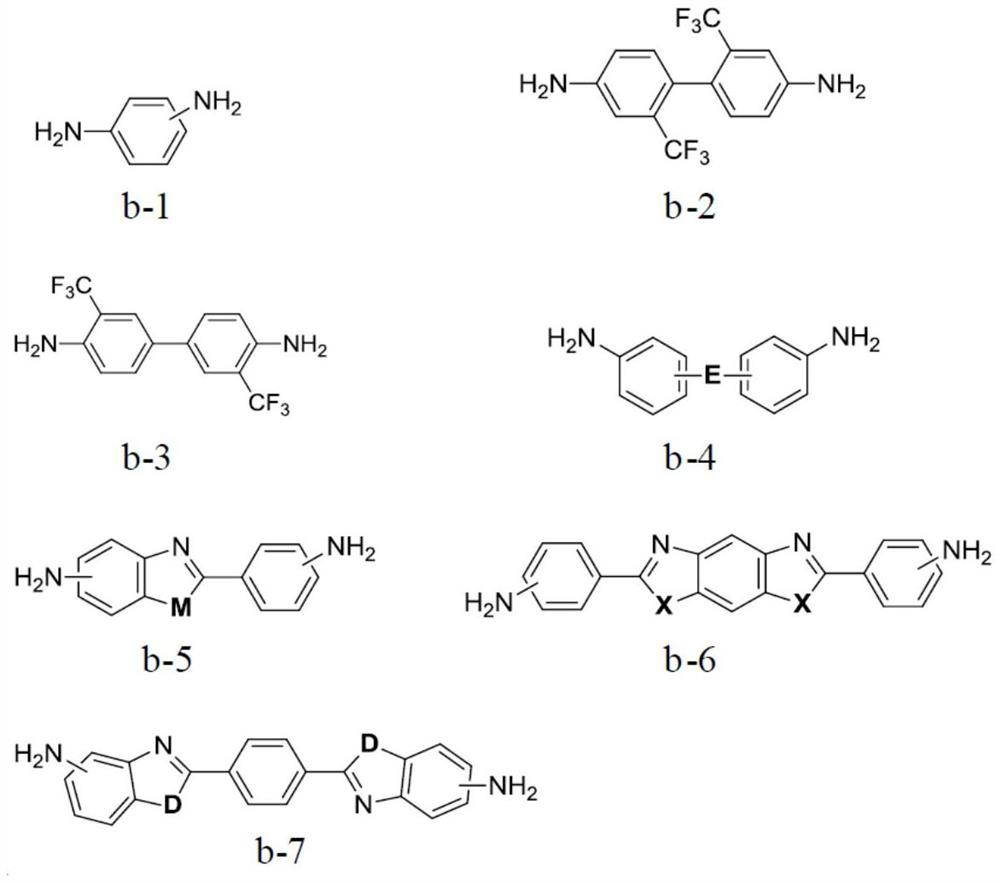
[0060] The monomer B is selected from one or more of the structures shown in formula b-1 to formula b-7:
[0061]
[0062] in,
[0063] M is selected from -O-, -S-, or -NH-;
[0064]X is selected from -O-, -S-, or -NH-;
[0065] D is selected from -O-, -S-, or -NH-;
[0066] E is selected from -O-, -S-, -SO 2 -, -CH 2 -, -C(CF 3 ) 2 -, -CO-,...
preparation example 1
[0203] Raw material preparation example 1 Preparation of diamine shown in monomer A-formula a-1
[0204] (1) 69.52 grams (0.56 moles) of 3-methoxyphenol, 88.23 grams (0.56 moles) of p-nitrochlorobenzene, 85.14 grams (0.616 moles) of potassium carbonate and 200 grams of dimethyl sulfoxide were added to the reactor in sequence , heated to 160°C for 6 hours; cooled to 60°C, added to 2000 ml of water, the crude product was precipitated, filtered out, washed with water, dissolved in dichloromethane, dried over anhydrous magnesium sulfate, and concentrated to obtain the crude product 114.23 grams of refined product of compound (II-1) represented by formula (II) were obtained through recrystallization; the yield was 83.2%.
[0205] Utilize nuclear magnetic resonance to characterize the structural compound (II-1) shown in the formula (II) that obtains, the proton nuclear magnetic resonance spectrum result that obtains is: 1 H NMR (400MHz, DMSO) δ = 8.280–8.205 (m, 2H), 7.389 (t, J = 8....
preparation example 2
[0212] Raw material preparation example 2 Preparation of diamine shown in monomer A-formula a-2
[0213] (1) 62.07 grams (0.50 moles) of 3-methoxyphenol, 101.0 grams (0.50 moles) of m-bromonitrobenzene, 4.76 grams (0.025 moles) of cuprous iodide, 76.02 grams (0.55 moles) of potassium carbonate and 200 Add grams of N,N-dimethylformamide to the reaction flask in turn, heat the reaction system to 150°C under nitrogen protection and react for 12 hours; after cooling to 60°C, add it to 2000 ml of water, precipitate the crude product, filter it out, and wash with water Afterwards, it was dissolved in dichloromethane, dried over anhydrous magnesium sulfate, the solvent was concentrated, and purified to obtain 90.59 g of the refined product of the structural compound (II-2) shown in formula (II); the yield was 73.9%.
[0214] (2) 89.07 grams (0.48 moles) of 4-nitrobenzoyl chloride, 69.34 grams (0.52 moles) of aluminum trichloride, 1000 milliliters of dichloromethane and 105.45 grams (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com