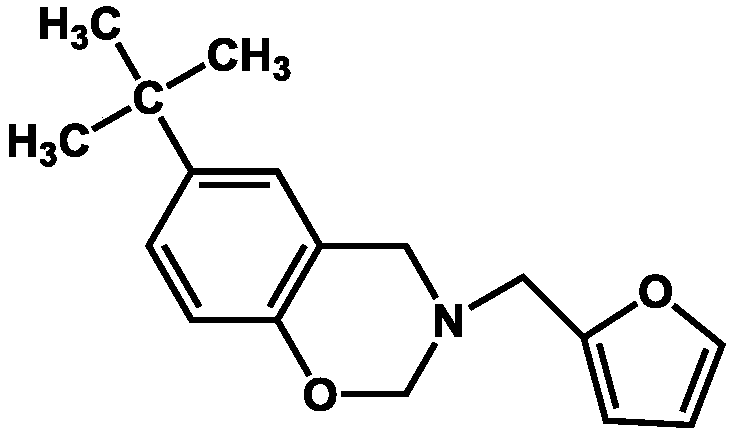
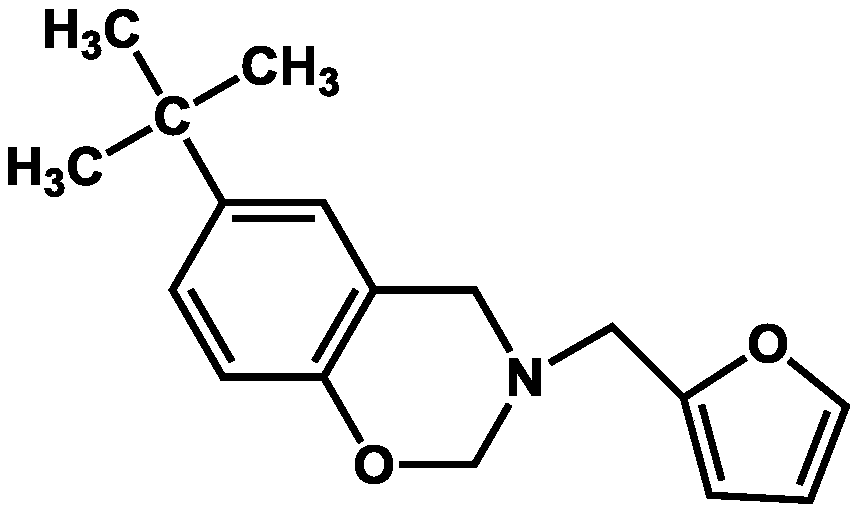
Tert-phenol-furfuryl amine type benzoxazine monomer, cured resin, and preparation method of copolymerized resin thereof
A benzoxazine and furfurylamine type technology, applied in the field of organic polymer materials, can solve problems such as not being able to meet requirements well, achieve good thermal performance, reduce dielectric constant and dielectric loss, and improve thermal performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Under nitrogen protection, add 0.1mol p-tert-butylphenol, 0.1mol furfurylamine, and 0.2mol paraformaldehyde into a 250mL three-necked flask equipped with a condenser, magnetic stirring, and thermometer. The molar ratio is 2:1:1, then add 51mL xylene / butanone mixed solvent (the volume ratio is 3:1), mix well and heat to 80°C for 48h reaction, after the reaction, pour the reaction solution into 100mL methanol solution (concentration 80wt%) to obtain a suspension, let it stand for 24 hours, remove the supernatant to obtain a precipitate, dry the precipitate at 60°C for 8 hours in vacuum, and finally grind the dried product to obtain a powder that is tert-phenol-furfurylamine type benzoxazine monomer.
Embodiment 2
[0031] Under nitrogen protection, add 0.1mol furfurylamine and 0.2mol paraformaldehyde into a three-necked flask equipped with a condenser, magnetic stirring, and a thermometer, add 60mL of chloroform / methanol mixed solvent (the volume ratio is 1: 1), and mix After stirring for 1.5h, add 0.1mol p-tert-butylphenol, the molar ratio of aldehyde group, phenolic hydroxyl group and amino functional group is 2:1:1, heat to 120°C and continue the reaction for 4.5h, after the reaction is completed, pour the reaction solution into 100mL Obtain a suspension in a methanol solution (concentration 65wt%), let it stand for 24 hours, remove the supernatant to obtain a precipitate, dry the precipitate in vacuum at 60°C for 6 hours, and finally grind the dried product to obtain a powder that is tert-phenol-bran Amine type benzoxazine monomer.
Embodiment 3
[0033]Under the protection of nitrogen, add 0.1mol furfurylamine and 0.2mol paraformaldehyde into a three-necked flask equipped with a condenser, magnetic stirring, and a thermometer, and add 60mL of dioxane / ethyl acetate (the volume ratio is 1: 3 ), after mixing and stirring for 2 hours, add 0.1mol of p-tert-butylphenol, the molar ratio of aldehyde group, phenolic hydroxyl group and amino functional group is 2:1:1, heat at 110°C and continue to react for 12 hours, after the reaction, pour the reaction solution into 100mL Obtain a suspension in a methanol solution (concentration 70wt%), let it stand for 24 hours, remove the supernatant to obtain a precipitate, dry the precipitate in vacuum at 60°C for 12 hours, and finally grind the dried product to obtain a powder that is tert-phenol-bran Amine type benzoxazine monomer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com