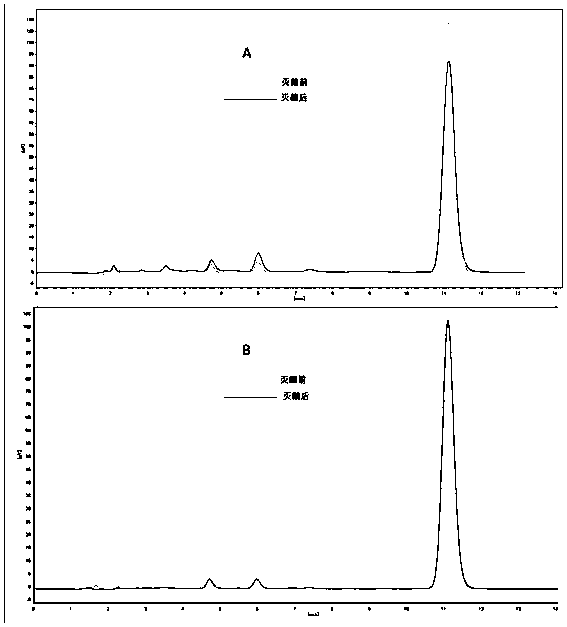
Vitamin K1 injection and preparation method thereof
A technology for vitamins and injections, applied in anti-inflammatory agents, pharmaceutical formulas, non-central analgesics, etc., can solve the problems of poor stability, easy decomposition, and darkening of vitamin K1, and is suitable for industrial scale-up production, improving solubility, The effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1 Preparation of Vitamin K1 Mixed Micelle Solution and Injection
[0068] The specification of the injection to be prepared in this example is 1mL / bottle, each containing 10mg of the main drug vitamin K1, calculated on the basis of 1000 injections, the composition is as follows:
[0069]
[0070] The specific operation process is as follows:
[0071] (1) Weigh each component according to the stated ratio, put it in a beaker, add an appropriate amount of ethanol, put it in a water bath magnetic stirrer, heat to 40°C ± 2°C, stir, and dissolve until clear;
[0072] (2) heat the solution obtained in step (1) at 40°C ± 2°C, keep it away from light, pass nitrogen gas for 1-2 hours, and volatilize to remove ethanol to obtain a semi-solid mixture;
[0073] (3) Add 80% of the prescribed amount of water for injection to the semi-solid mixture obtained in step (2), adjust the pH to 5.4, continue to stir until clarified, make up the amount of water for injection to the ...
Embodiment 2
[0075] The physicochemical property test of embodiment 2 micelle solution
[0076] (1) Particle size and Zeta potential measurement
[0077] The solution containing vitamin K1 mixed micelles prepared in Example 1 was diluted to 0.5 mg / mL, and the average particle size of the micelles was measured by MalvernNano ZS90 Zetasizer, and the average particle size of the micelles was 9.7±2.0 nm.
[0078] (2) Morphological investigation
[0079] The diluted mixed micelle solution was added dropwise to the copper grid, counterstained with 2% sodium phosphotungstate solution, observed and photographed under a transmission electron microscope (TEM). The prepared mixed micelles are evenly distributed quasi-spherical entities (such as Figure 7 shown).
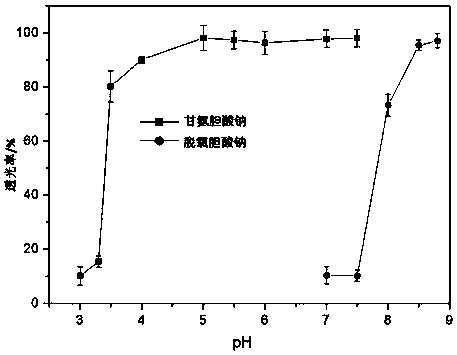
[0080] (3) Solution light transmittance test
[0081] Take the mixed micelle solution, and measure its light transmittance (%) at 600 nm by using a UV-visible spectrophotometer. The light transmittance of the solution was measured to b...
Embodiment 3
[0090] The 2-year stability study of embodiment 3 injection
[0091] The sterilized injection was prepared according to the method of Example 1, and a 2-year stability study was carried out, and the results are shown in Table 1.
[0092] Table 1 Stability data of vitamin K1 phospholipid / bile salt mixed micelles sterilized injection
[0093]
[0094] As can be seen from Table 1, the injection solution of the present invention has good stability, and the storage period can reach more than 2 years.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com