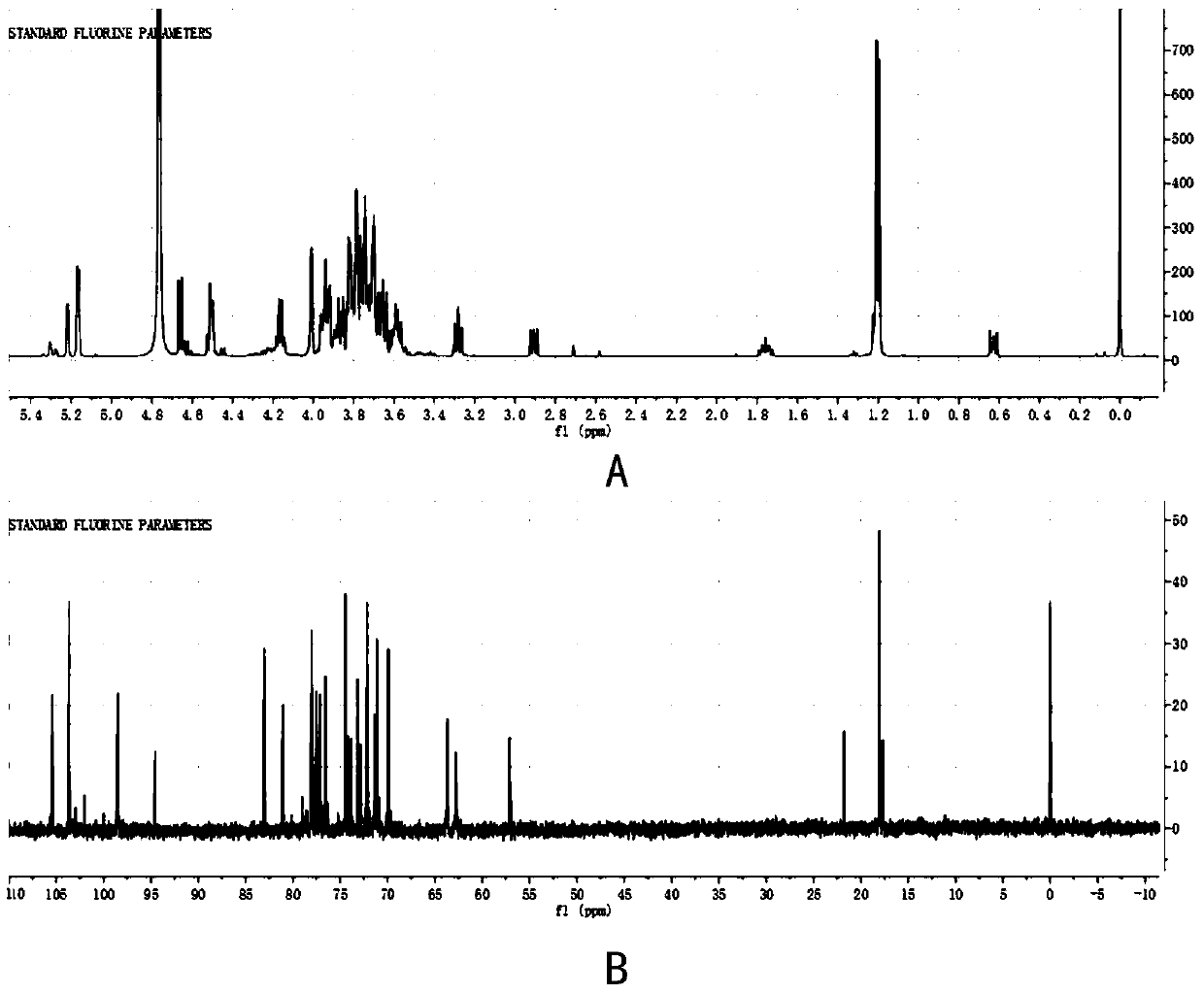
Alpha-L-fucosidase, and related biological material and application thereof
A technology of fucosidase and biomaterials, applied in the field of genetic engineering, can solve the problems of little functional activity of lactose and no research reports on the probiotic activity of 3'-fucosyllactose
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1: Construction of recombinant α-L-fucosidase encoding gene expression plasmid
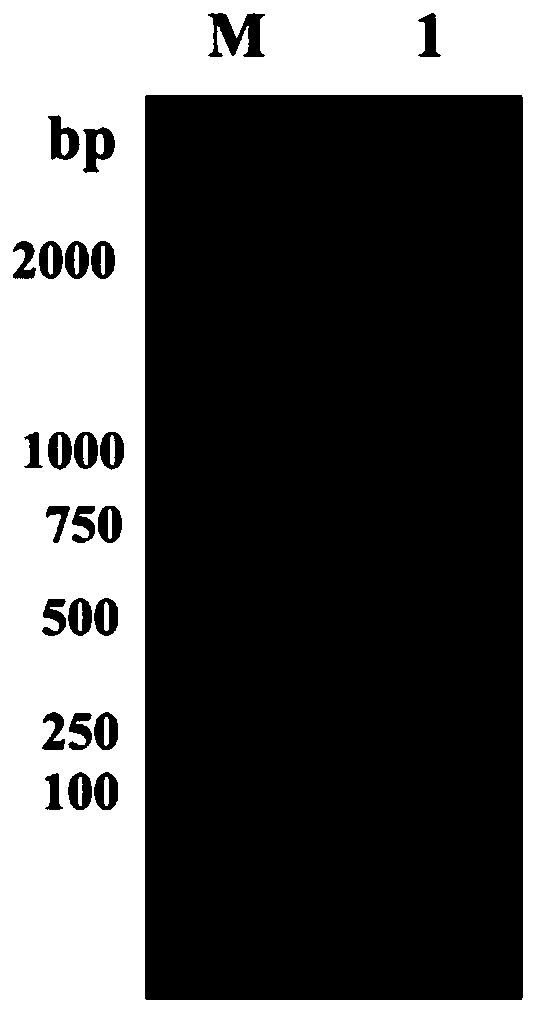
[0084] 1. Extract the genomic DNA of Pedobacter sp. and use it as a template, artificially synthesized degenerate primers fuDP-F: 5′-ACNACNAARCAYCAYGAYGGNTTY-3′ and fuDP-R: 5′-RTTNACNARCATRTTNCCCNCC-3′ Primers, carry out gradient PCR amplification (annealing temperature is 40 ℃ ~ 60 ℃), the amplification product is detected by 1% agarose gel electrophoresis, and a conserved sequence of about 700bp is obtained. The results are as follows figure 1 .
[0085] Gradient PCR amplification system: 10×LA buffer 5.0μl, dNTP mix (2.5mmol / 1) 4.0μl, fuDP-F / R (10pmol / μl) each 8.0μl, genomic DNA 1.0μl, LA Taq (5.0U / pl) 0.5 μl, ddH 2 O up to 50.0 μl; gradient PCR amplification program: 95°C pre-denaturation for 3 minutes; 95°C denaturation for 30 seconds, 40-60°C annealing for 30 seconds, 72°C extension for 40 seconds, 34 cycles; 72°C for 5 minutes.
[0086] 2. Ligate the amplified conserved s...
Embodiment 2
[0102] Embodiment 2: Expression of recombinant α-L-fucosidase gene
[0103] 1. Construction of recombinant strains and expression of recombinant α-L-fucosidase
[0104] The recombinant plasmid pET-28a(+)-PbFuc was transformed into Escherichia coli BL21(DE3) to obtain a recombinant bacterium, which was named BL21(DE3)-pET-28a(+)-PbFuc. Insert BL21(DE3)-pET-28a(+)-PbFuc into LB liquid medium for seed liquid culture, medium containing kanamycin (50 μg mL -1 ), the seed liquid inoculum size is 1.5% (w / v), and the solid medium is an LB solid plate containing agar. Pick positive transformants from the solid medium plate into liquid medium, culture at 37°C for 12 hours, transfer to 200mL LB medium with 1% inoculum size, culture at 37°C, when the culture medium is OD 600 When it reaches 0.6-0.8, add IPTG (with IPTG as the experimental group, without IPTG as the control group) to a final concentration of 1mmol L -1 , Induced at 20°C for 16h, the cells were collected by centrifugatio...
Embodiment 3
[0113] Example 3: Enzymatic properties of recombinant α-L-fucosidase (PbFuc)
[0114] 1. Definition and determination method of PbFuc enzyme activity
[0115] α-L-fucosidase activity was determined with reference to the method of Janet et al. (Janet et al., α-Fucosidase with different substrate specificities from two species of Fusarium. Appl Microbiol Biotechnol, 2013, 97: 5371-5380.). Add 100 μL 10mM pNP-FUC, 100 μL 0.05M pH 5.0 citric acid-trisodium citrate buffer, 10 μL appropriately diluted enzyme solution, react at 35°C for 20 min, and finally add 200 μL Na 2 CO 3 (1M) Stop the reaction and shake evenly. Take 200 μL and add it to a 96-well plate, and measure the absorbance at 405 nm. The pNP standard was used as a standard curve. Enzyme activity definition: The amount of enzyme required to catalyze pNP-FUC to generate 1 μmol pNP per minute is one enzyme activity unit (U).
[0116] 2. Enzymatic properties of PbFuc
[0117] (1) Optimum reaction pH and pH stability of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com