Multiplex detection kit for EGFR gene mutation
A detection kit and multiple detection technology, applied in the field of biotechnology and tumor diagnosis, can solve the problems of low sensitivity, long time, and accuracy of small fragment deletion.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0134] The invention provides a method for detecting multiple gene mutations in lung cancer, primers, probes and a kit. The specific implementation steps are as follows:
[0135] (1) Extraction of nucleic acid templates from samples to be tested
[0136] Nucleic acid extraction or purification reagents from Sun Yat-Sen University Daan Gene Co., Ltd. (Tissue samples can use Yuesui Machinery Equipment No. 20170666; ascites and plasma samples can use Yuesui Machinery Equipment Number 20180628), and nucleic acid extraction is performed according to the kit instructions. The nucleic acid after tissue sample extraction is diluted to a concentration of 2-100ng / μL (the concentration of nucleic acid extracted from pleural fluid and plasma is not less than 2ng / μL), and the purity should meet A 260 / A 280 The ratio ranges between 1.7-2.3. The template can be used directly for subsequent experiments or stored at -80°C for future use, avoiding repeated freezing and thawing.
[0137] (2...
Embodiment 2
[0156] Embodiment 2 Sensitivity and accuracy detection
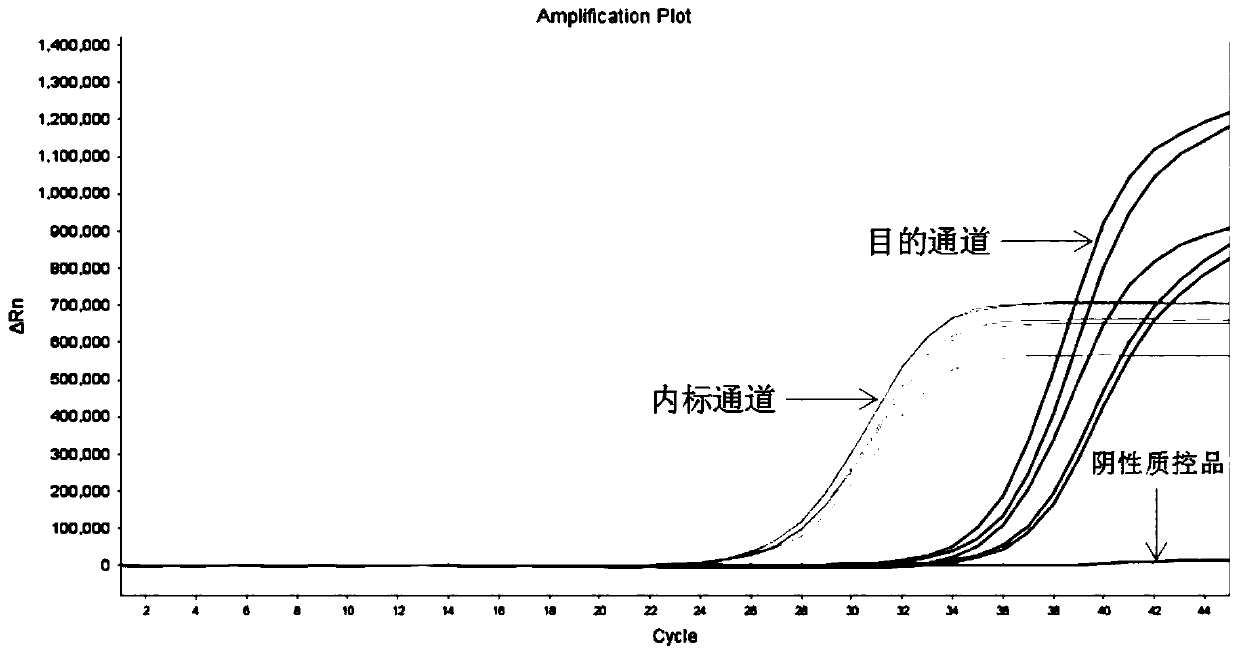
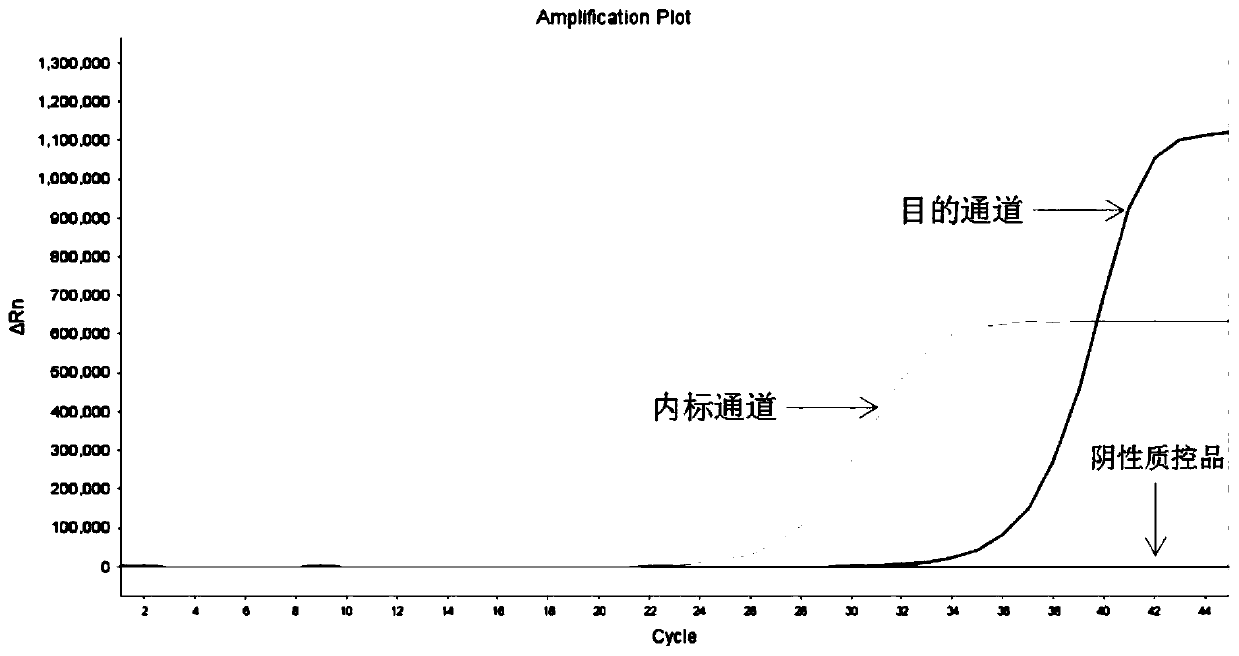
[0157] The sensitivity reference product with a nucleic acid concentration of 2 ng / μL is composed of detection gene locus plasmid DNA or cell line, mixed with wild-type cell line nucleic acid in a certain proportion, and the gene mutation rate of the mixture is 1%.
[0158] (1) Sample addition
[0159] Take 3 μL of the DNase system and add it to the PCR reaction tube. Centrifuge briefly for 15 s.
[0160] Take 5 μL of negative quality control substance, sensitivity reference substance (with a concentration of 2 ng / μL mutation rate of 1%), and positive quality control substance, and add them to the reaction tubes in sequence according to Table 5. Close the cap of the PCR reaction tube tightly, mix well, and centrifuge briefly for 10 s.
[0161] For each sensitivity reference product, 5 repeated experiments.
[0162] (2) Real-time fluorescent PCR amplification:
[0163] Set the real-time fluorescent PCR amplification ...
Embodiment 3
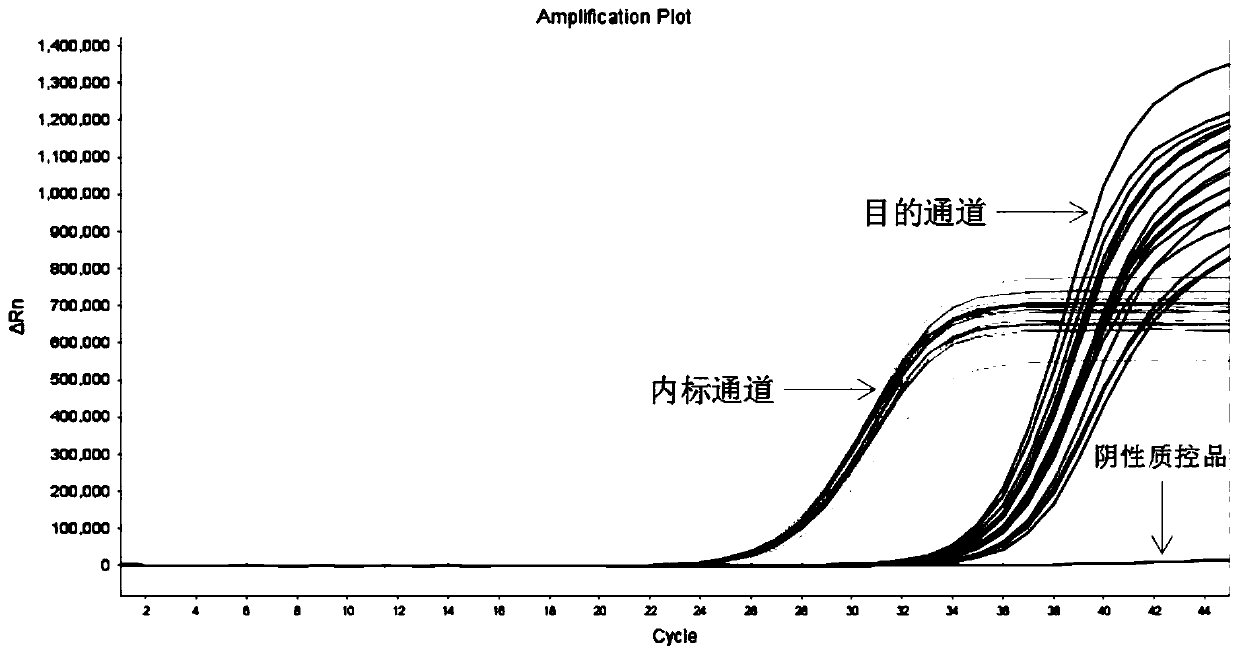
[0171] Embodiment 3 clinical sample detection
[0172] Extraction of test sample nucleic acid:
[0173] (1) Extraction of nucleic acid templates from clinical samples to be tested
[0174] 100 negative clinical samples were collected, and 1 case each of the 28 EGFR gene mutation clinical samples confirmed by conventional methods was randomly mixed in after numbering. Nucleic acid extraction or purification reagents from Sun Yat-Sen University Daan Gene Co., Ltd. (Tissue samples can be used in Guangdong Sui Ji Bei No. 20170666; ascites and plasma samples can use Yue Sui Ji Bei No. 20180628), and nucleic acid extraction was carried out according to the kit instructions, and the nucleic acid after tissue sample extraction was diluted to a concentration of 2-100 ng / μL (ascites and plasma extraction The concentration of nucleic acid is not less than 2ng / μL), and the purity should meet A 260 / A 280 The ratio ranges between 1.7-2.3. The template can be used directly for subsequen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com