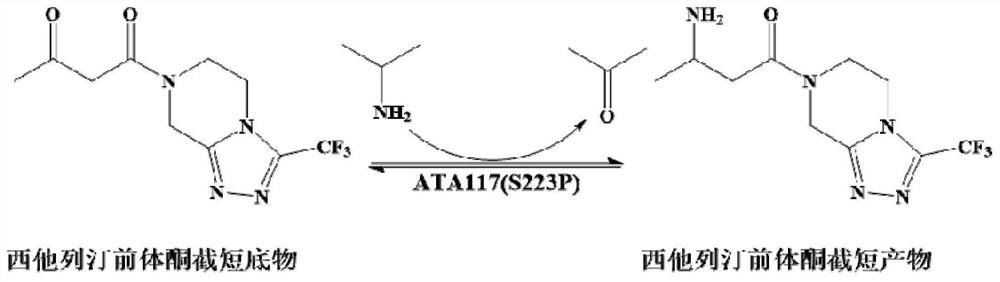
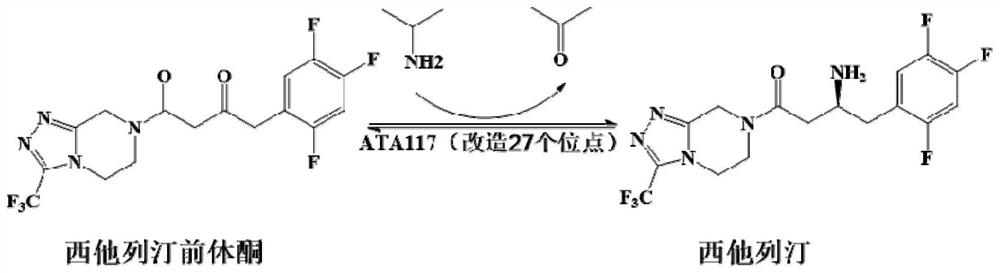
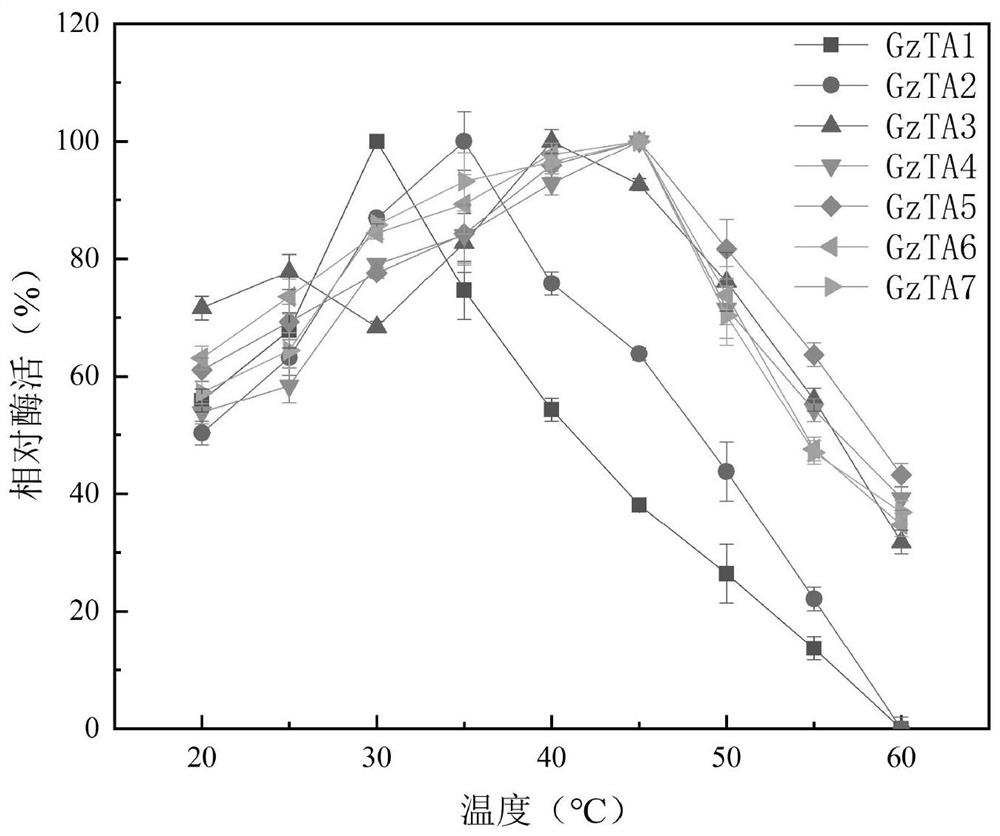
Recombinant r-ω-transaminase, mutant and its application in asymmetric synthesis of sitagliptin
A technology for sitagliptin and mutants, applied in the field of recombinant R-ω-transaminase, mutants and asymmetric synthesis of sitagliptin, to achieve high stereoselective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Screening of novel ω-TA, determination of stereoselectivity and precise determination of enzyme activity
[0027] 1. Enzyme source and gene synthesis
[0028] Using the amino acid sequence of the commercial enzyme R-ω-TA 117 as a template, three ω-TA strains were obtained by gene mining from the NCBI database, namely Gibberella zeae TA (GzTA, GenBank No. XP_381942.1), Mycobacterium vanbaalenii TA (MvTA, GenBank No. WP_011781668.1) and Neosartorya fischeri TA (NfTA, GenBank Accession XP_001261640.1). The homology between the above three enzymes and R-ω-TA 117 is 44%, 52% and 38%, respectively. Codon optimization was carried out according to the codon preference of E.coli, and the nucleotide sequences of the three enzymes were synthesized by the method of whole gene synthesis, which are respectively represented by SEQ ID NO.2, SEQ ID NO.4 and SEQ ID NO.6 shown; the amino acid sequences encoding the enzymes are shown in SEQ ID NO.1, SEQ ID NO.3 and SEQ ID NO.5...
Embodiment 2
[0043] Example 2: Construction and screening of GzTA single point mutants
[0044] 1. Mutant construction
[0045] Carry out single point mutation to the novel R-omega-TA screened, according to the amino acid sequence of NCBI's GzTA (GenBank number is XP_381942.1, the amino acid sequence is shown in SEQ ID NO: 1, and the nucleotide sequence is SEQ ID NO : 2 shows) design the mutation primer of site-directed mutation, utilize fast PCR technique, take recombinant vector pET28b / GzTA as template, introduce single mutation to the 60th position of GzTA amino acid sequence, primer is:
[0046] Forward primer GACCTGACCTACGAC NNK CCAGCTGTGTGGGAC (the underline is the mutated base)
[0047] Reverse primer GTCCCACACAGCTGG MNN GTCGTAGGTCAGGTC (the underline is the mutated base)
[0048] PCR reaction system: 2×Phanta Max Buffer (containing Mg 2+ ) 25μL, dNTPs 10mM, forward primer 2μL, reverse primer 2μL, template DNA 1μL, Phanta Max Super-Fidelity DNA Polymerase 50U, add ddH 2 0 to...
Embodiment 3
[0062] Example 3: Construction and screening of GzTA two-site mutants
[0063] The single mutant GzTA constructed according to Example 2 1 Sequence design of mutation primers for site-directed mutagenesis, using rapid PCR technology to recombinant vector pET28b / GzTA 1 as template, for GzTA 1 A single mutation is introduced at position 113 of the amino acid sequence, and the primers are:
[0064] Forward primer ATCAAAGACGCT NNK GTTGAACTGATCGTT (the underline is the mutant base)
[0065] reverse primer GATCAGTTCAAC MNN AGCGTCTTTGATACC (the underline is the mutated base)
[0066] The PCR reaction system is the same as the "construction of mutants" in Example 2.
[0067] The PCR amplification conditions were 95°C for 3min; (95°C for 15s, 50°C for 15s, 63°C for 6.5min) for 30 cycles; 72°C for 5min.
[0068] The PCR product was transformed into E.coli BL21(DE3) competent cells, and a single clone was picked in LB liquid medium containing 50 μg / mL kanamycin, and cultured ove...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com