Method for preparation of aromatic alcohol by photocatalysis of aromatic aldehyde conversion
A technology for aromatic aldehydes and aromatic alcohols, applied in the field of photocatalytic asymmetric synthesis, can solve the problems of limited raw materials, high cost, harsh reaction conditions, etc., and achieve the effects of short reaction cycle, high conversion rate and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The present embodiment provides a kind of photocatalytic aromatic aldehyde conversion and prepares the method for aromatic alcohol, and it comprises the following steps:
[0022] (a) Mix 5mg / mL titanium dioxide photocatalyst (P25, commercially available, Degussa), 10mmol benzaldehyde and 2mL methanol evenly, and disperse for 30min by ultrasonic (power 80W) to obtain a suspension;
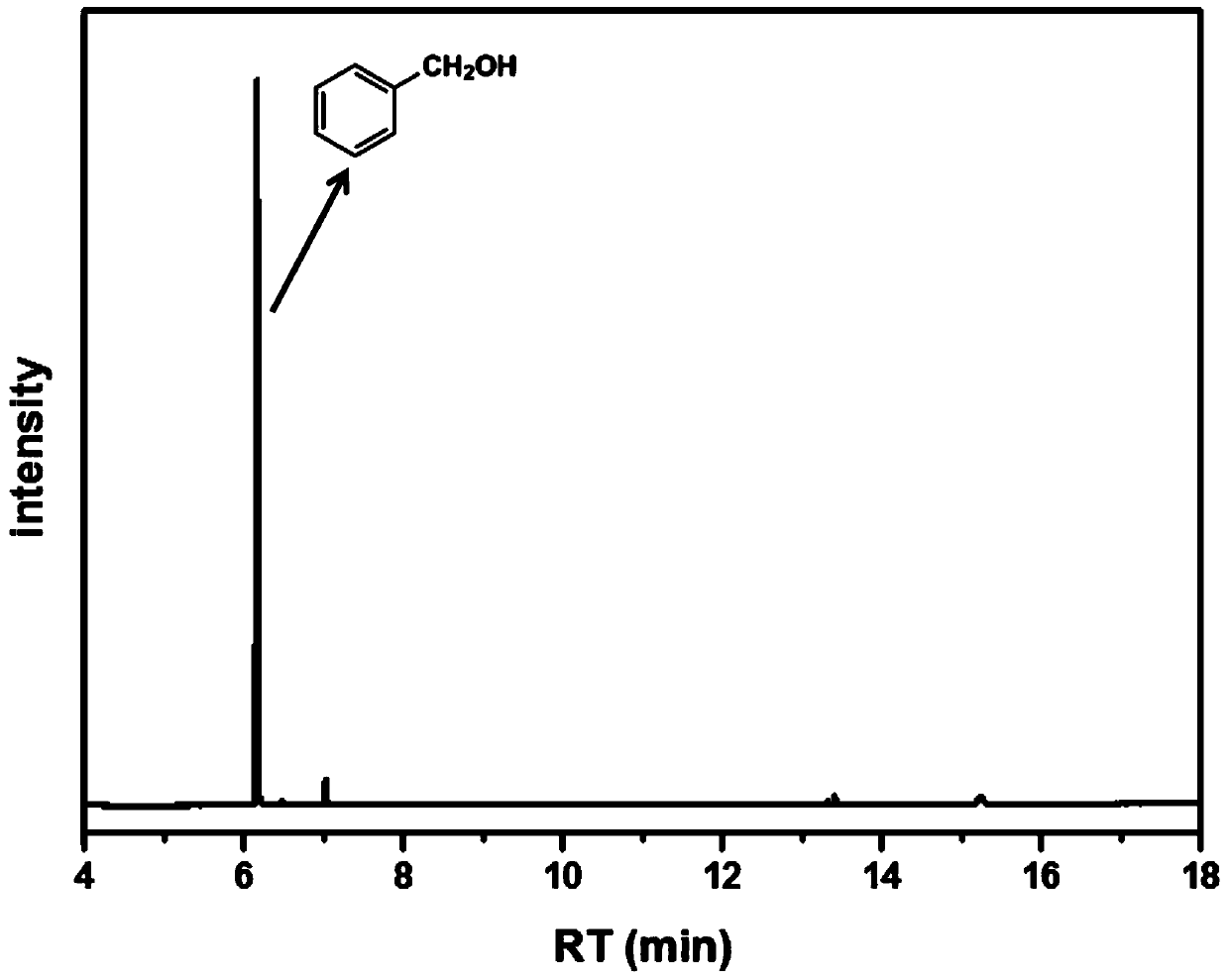
[0023] (b) The dispersed suspension is irradiated by LED lamps simulating sunlight (the wavelength of light is 365nm, and the light power is 2.0W / cm 2 ) and nitrogen protection, stirred at room temperature and reacted for 2h to obtain a reactant solution (containing benzyl alcohol, the reactant solution was tested by gas chromatography to obtain figure 1 As shown in the collection of illustrative plates, it can be seen that the benzaldehyde peak at 5.3min cannot be observed substantially; that is, the conversion rate of benzaldehyde is 100%);
[0024] (c) reactant solution is extracted (get ...
Embodiment 2
[0026] The present embodiment provides a kind of photocatalytic aromatic aldehyde conversion and prepares the method for aromatic alcohol, and it is basically consistent with embodiment 1, and difference is: in step (a), photocatalyst is acid treatment (specifically: the 0.5 g P25 was dispersed in 50mL of deionized water, 1mL of nitric acid was added dropwise to the above solution, and then the solution system was placed in an ultrasonic cleaner with an electric power of 100W for 1h, taken out and centrifuged and washed twice with deionized water and ethanol respectively. Acid-treated P25); the conversion rate of final benzaldehyde is 100%, and the selectivity of benzyl alcohol is 87.7%.
Embodiment 3
[0028] The present embodiment provides a kind of photocatalytic aromatic aldehyde conversion and prepares the method for aromatic alcohol, and it is basically consistent with embodiment 1, difference is: in step (a), photocatalyst is Ag / P25 composite catalyst (Ag accounts for composite catalyst The ratio is 1wt%); The final conversion rate of benzaldehyde is 100%, and the selectivity of benzyl alcohol is 87.8%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap