Double-color fluorescent probe as well as synthesis method and application thereof
A dual-color fluorescent probe and fluorophore technology, applied in the field of dual-color fluorescent probes and their synthesis, achieves the effects of low cytotoxicity, simple preparation and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] In another specific embodiment of the present invention, a preparation method of the above-mentioned dual-color fluorescent probe is provided, and the preparation method includes the following reactions:
[0034]
[0035] In another specific embodiment of the present invention, the step a is specifically:
[0036] Under the protection of inert gas, bromopropylamine, DPA and potassium carbonate are mixed and stirred to obtain a light yellow solid A. A.
[0037] In another specific embodiment of the present invention, the step b is specifically:
[0038] Under the protection of inert gas, mix caffeic acid, DMAP, DCC, triethylamine and DMF, and stir under ice bath conditions to obtain an activated caffeic acid solution;
[0039] Under the protection of inert gas, after mixing ethylenediamine and DMF, the activated caffeic acid solution was added dropwise, stirred at room temperature to obtain a brown turbid liquid, and thin layer chromatography to obtain a light yellow solid B.
[0...
Embodiment 1
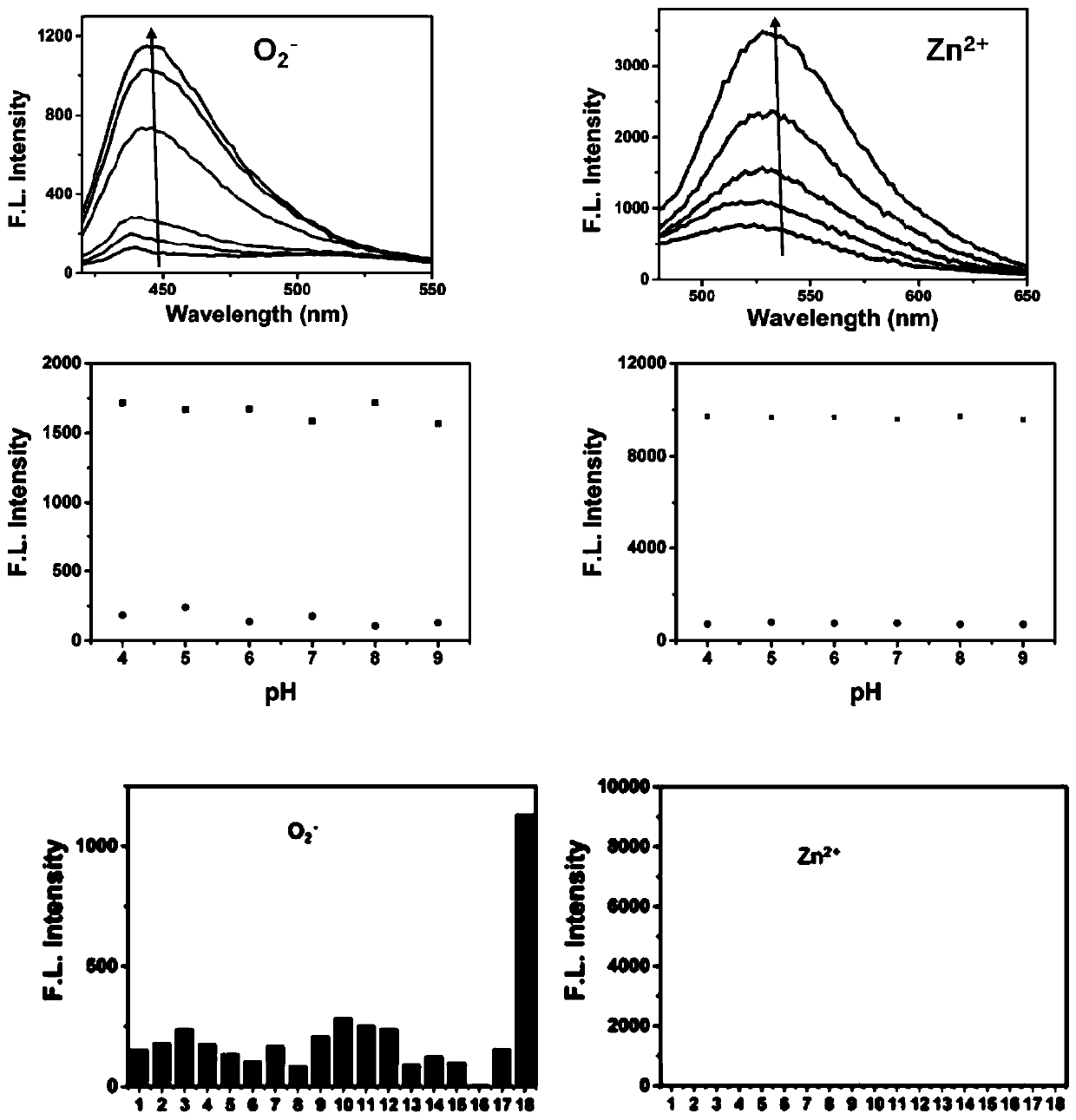
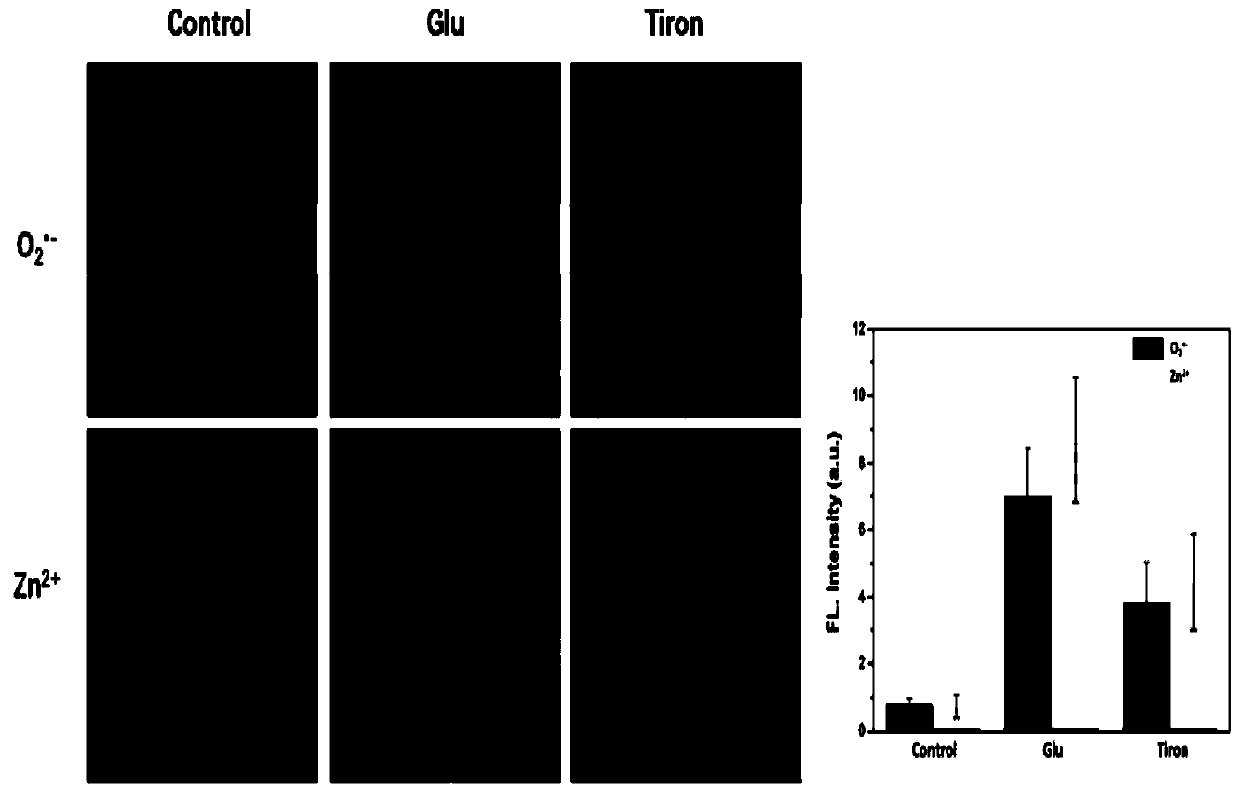
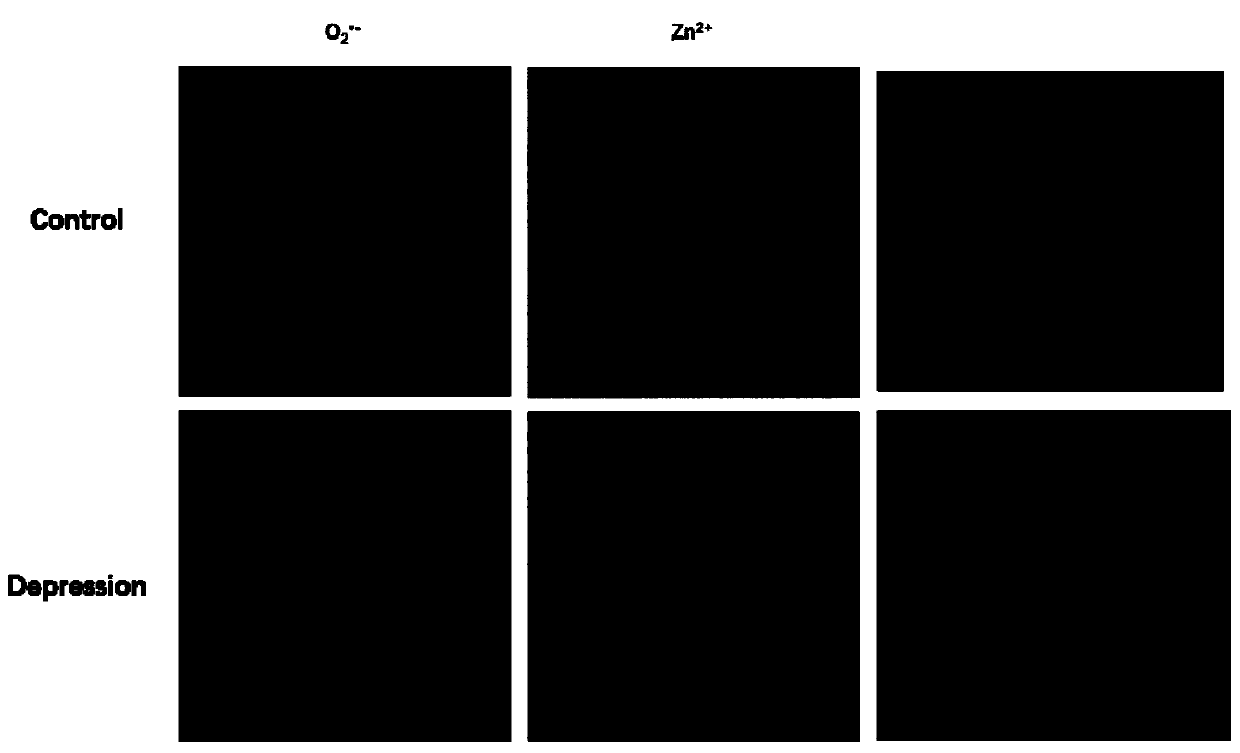
[0052] A detection cell and in vivo O 2 ·- And Zn 2+ The preparation method of the new fluorescent probe is as follows:
[0053]
[0054] Step a: Under nitrogen protection, add 2.18g bromopropylamine, 1.99gDPA, 0.50g K 2 CO 3 Put 30ml of acetonitrile into a two-necked flask, stir at room temperature for 12h, filter, and rotate to obtain a light yellow solid A. Mass spectrum see Figure 4A .
[0055]
[0056] Step b: Under the protection of nitrogen, put 1.8g caffeic acid, 1.2g DMAP, 2g DCC, 1.4ml triethylamine and 12ml DMF into a two-necked flask, and stir for 30 minutes under ice bath conditions to obtain an activated caffeic acid solution. Under nitrogen protection, 670 microliters of ethylenediamine and 5ml DMF were added to the three-necked flask, and then the activated caffeic acid solution was added dropwise to the three-necked flask, and stirred at room temperature for 12h to obtain a brown cloudy liquid. Thin layer chromatography (eluent: dichloromethane: methanol = 10:1) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com