Preparation method of terbutaline sulfate
A technology of terbutaline sulfate and sulfuric acid, which is applied in the field of medicine, can solve the problems of low yield of step factory, low yield of long step, and low yield, so as to avoid high-risk and highly toxic reagents and solve the problem of low yield of long step Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
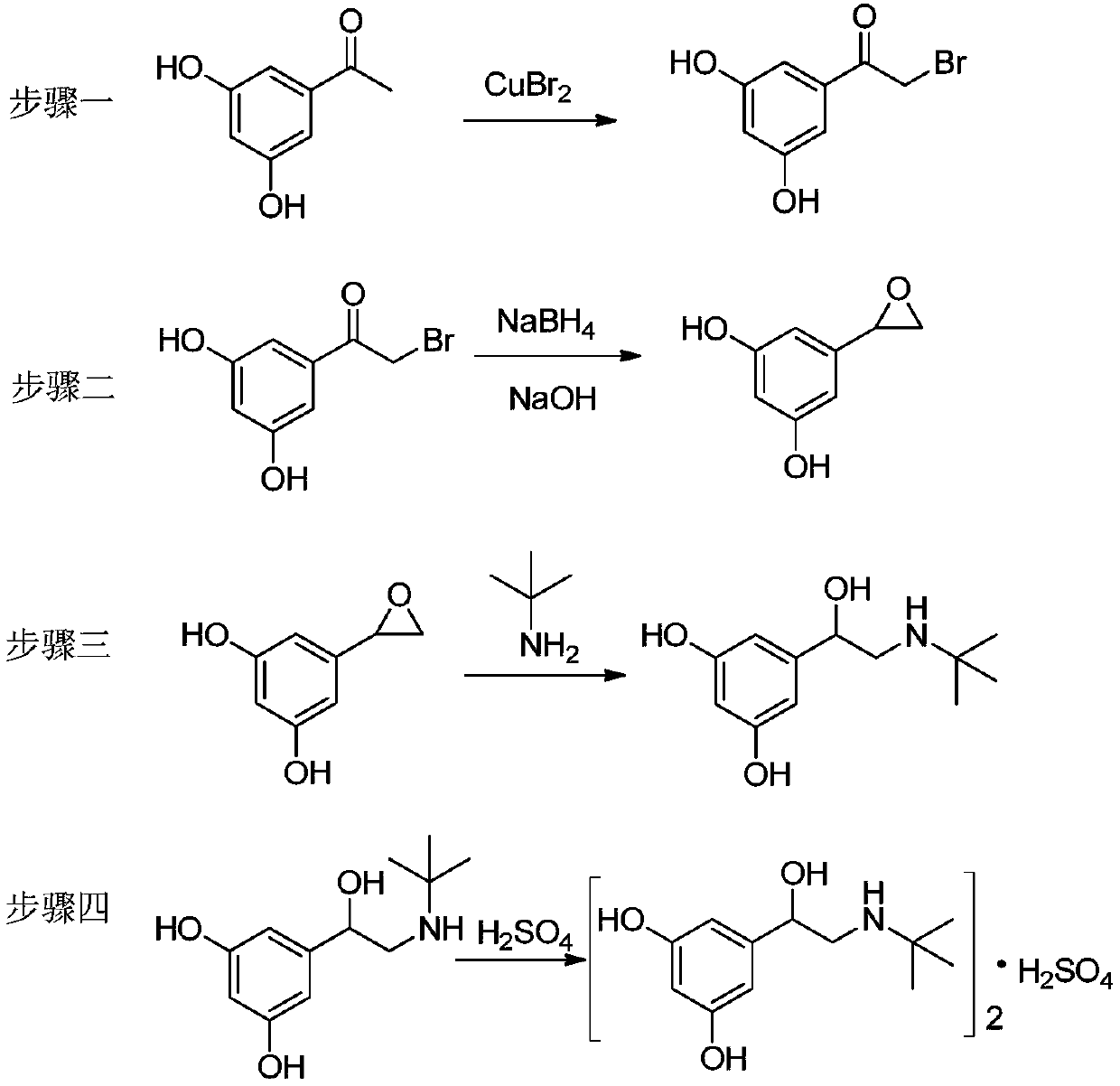
[0030] (1) Add 45.8g of copper bromide to 200ml of ethyl acetate, stir at room temperature, dissolve 15.5g of 3,5-dihydroxyacetophenone in 90ml of chloroform, add it dropwise to the reaction flask, and heat up to 70°C After reacting for 8 hours, it was cooled to room temperature, filtered with diatomaceous earth, and the filtrate was concentrated under reduced pressure to obtain 21.6 g of 5-bromoacetyl resorcinol;
[0031] (2) Dissolve 20.5g of 5-bromoacetyl resorcinol in 200ml of ethanol, cool in an ice-water bath to 10°C, add 3.1g of sodium borohydride in batches, then rise to 25°C for 2 hours, add 10% hydroxide 22ml of sodium solution, react at room temperature for 3h, cool down to 5°C, add 100ml of dilute hydrochloric acid to quench the reaction, add 200ml of dichloromethane for extraction, dry the organic phase over anhydrous sodium sulfate, filter, and concentrate the filtrate under reduced pressure to obtain 2-(3,5 -dihydroxyphenyl) oxirane 11.6g;
[0032] (3) Dissolve...
Embodiment 2
[0035] (1) Add 65.2g of copper bromide to 250ml of ethyl acetate, stir at room temperature, dissolve 30.8g of 3,5-dihydroxyacetophenone in 200ml of chloroform, add it dropwise to the reaction flask, and heat up to 70°C After reacting for 6 hours, it was cooled to room temperature, filtered with diatomaceous earth, and the filtrate was concentrated under reduced pressure to obtain 37.2 g of 5-bromoacetyl resorcinol;
[0036] (2) Dissolve 35.2g of 5-bromoacetyl resorcinol in 260ml of methanol, cool in an ice-water bath to 5°C, add 5.2g of sodium borohydride in batches, then rise to 25°C for 3 hours, add 15% hydroxide Potassium solution 30ml, react at room temperature for 3h, cool down to 5°C, add 120ml of dilute hydrochloric acid to quench the reaction, add 280ml of dichloromethane for extraction, dry the organic phase over anhydrous sodium sulfate, filter, and concentrate the filtrate under reduced pressure to obtain 2-(3,5 -dihydroxyphenyl)oxirane 18.1g;
[0037] (3) Dissolve...
Embodiment 3
[0040] (1) Add 58.3g of copper bromide to 250ml of ethyl acetate, stir at room temperature, dissolve 23.6g of 3,5-dihydroxyacetophenone in 120ml of chloroform, add it dropwise to the reaction flask, and heat up to 75°C After reacting for 5 hours, it was cooled to room temperature, filtered through diatomaceous earth, and the filtrate was concentrated under reduced pressure to obtain 31.8 g of 5-bromoacetyl resorcinol;
[0041] (2) Dissolve 30.1 g of 5-bromoacetyl resorcinol in 200 ml of isopropanol, cool in an ice-water bath to 5°C, add 6.9 g of potassium borohydride in batches, then rise to 20°C for 3.5 hours, add 10 % Potassium hydroxide solution 28ml, react at room temperature for 4h, cool to 5°C, add 100ml of dilute hydrochloric acid to quench the reaction, add 200ml of dichloromethane for extraction, dry the organic phase over anhydrous sodium sulfate, filter, and concentrate the filtrate under reduced pressure to obtain 2-( 3,5-dihydroxyphenyl) oxirane 14.3g;
[0042] (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com