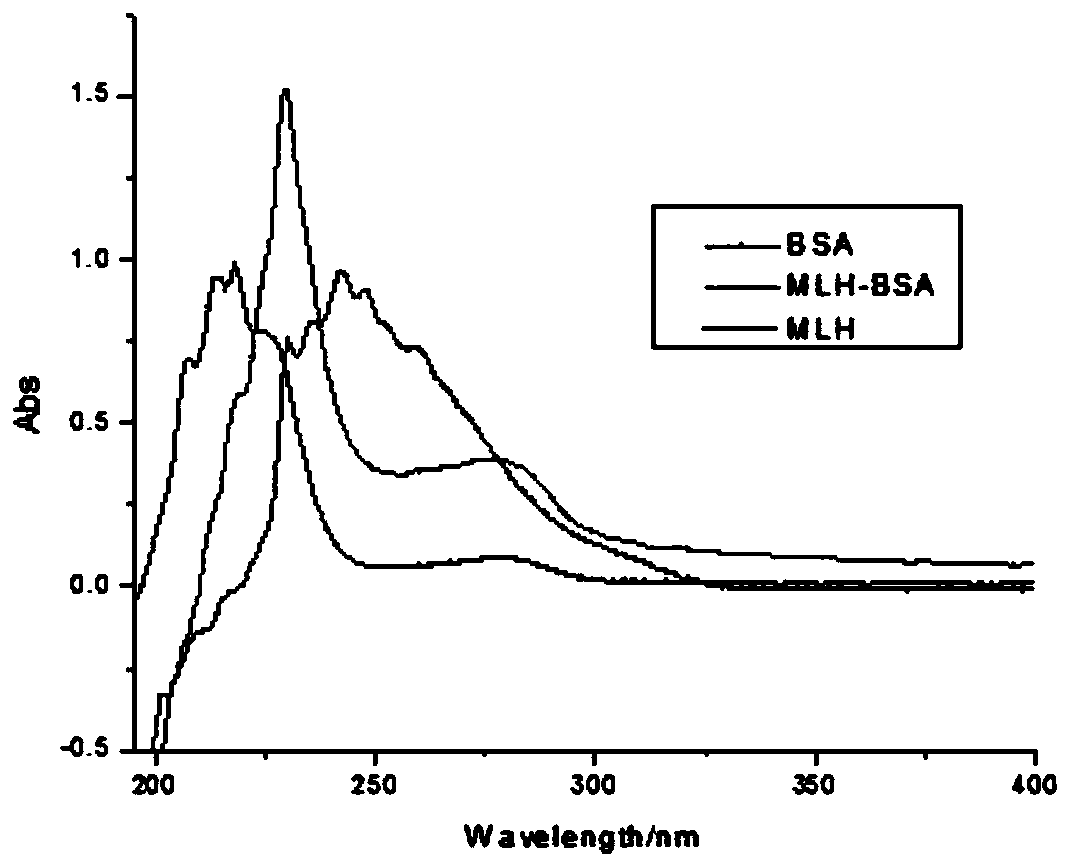
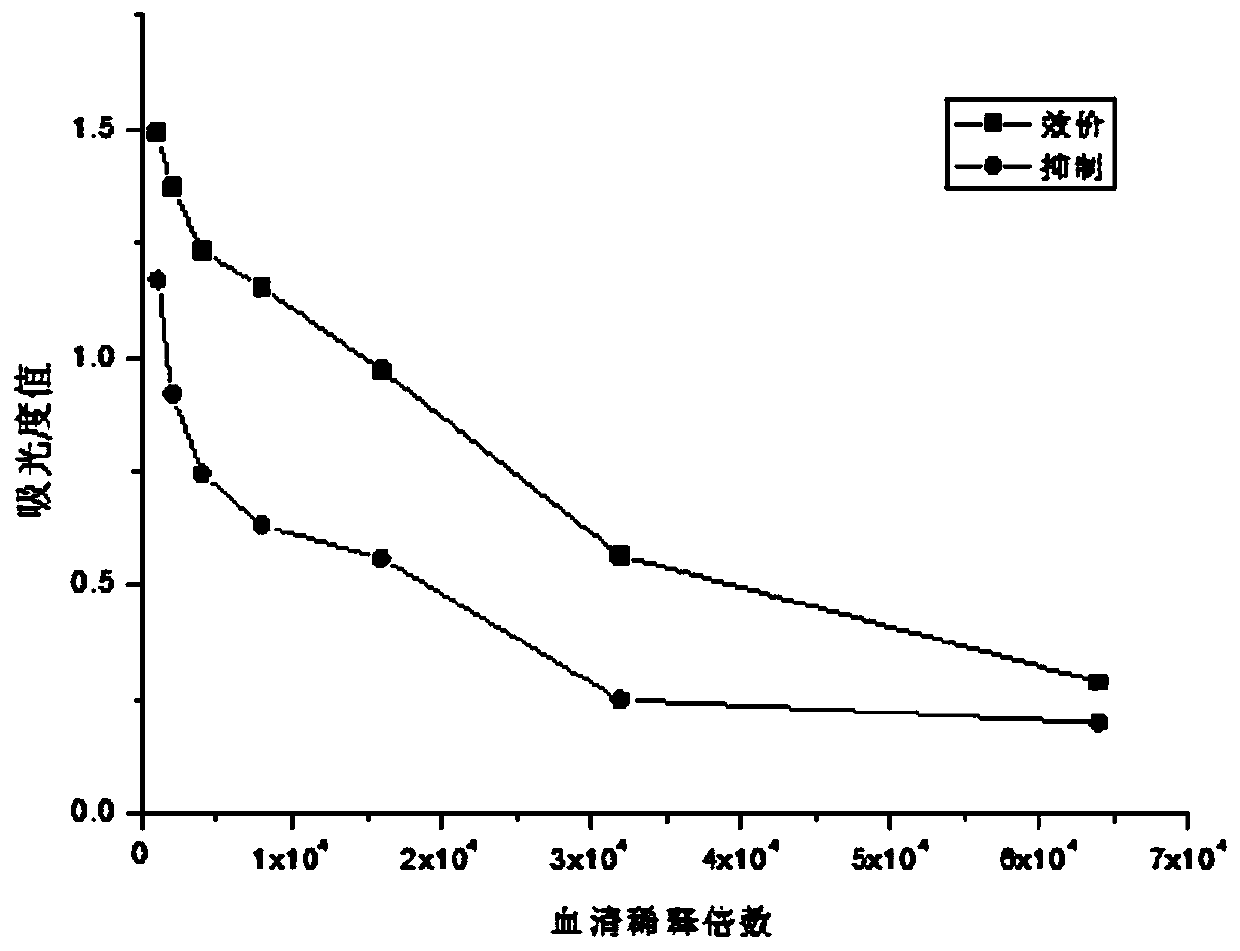
Malathion hapten and preparation method thereof, and malathion artificial antigen
A malathion and artificial antigen technology, applied in the field of food analysis, can solve the problems that hinder the development of immunological detection methods for malathion, complicated synthesis steps, and unreasonable hapten structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] The preparation of embodiment 1 malathion hapten MLH (n=5)
[0100] 1. Experimental steps
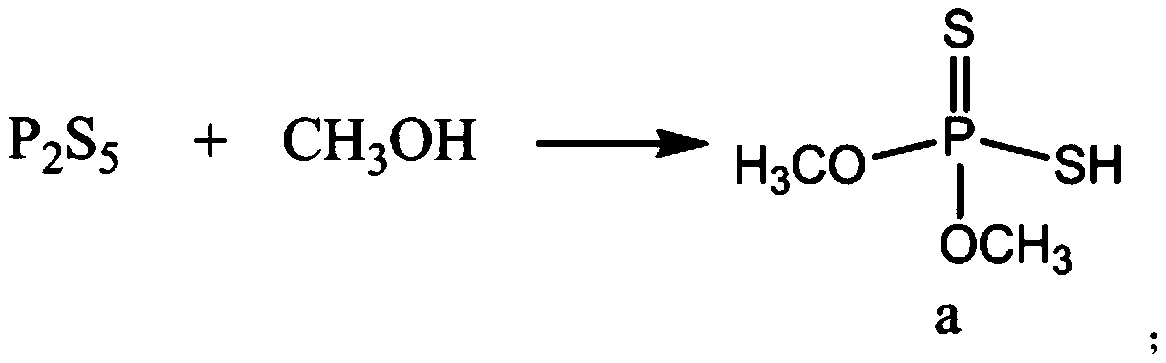
[0101] The preparation of the malathion hapten MLH (n=5), the synthetic route is as follows:
[0102]
[0103] Specific steps include:
[0104] 1.1 take 1g P 2 S 5 Put it into the reaction bottle, add 1.2mL of toluene, stir, heat up to 45°C, start to add 1mL of methanol dropwise at 0.2mL / min, slowly drop into the reaction bottle through the dropper, keep at 55±5°C, react for 2h, When cooling to below 40°C, take out the colorless crude sulfide with pungent smell;
[0105] 1.2 Take 0.948g of the crude sulfide described in step 1.1, add 6mmoL of ethyl hydrogen maleate, and after the addition, keep the reaction at 55°C for 1h, and keep it at 70-75°C for 8h to obtain the intermediate product b;
[0106] 1.3 Take 0.302mg (0.83mmoL) of the intermediate product b described in step 1.2, dissolve it in 0.2mL DMF solution, add 1mmoL DCC and NHS solution respectively, react at 4°C fo...
Embodiment 2
[0113] The preparation of embodiment 2 malathion artificial antigen MLH-BSA
[0114] 1. Experimental steps
[0115] The preparation of malathion artificial antigen, concrete steps comprise:
[0116] 1.1 Dissolve 2mmoL of the malathion hapten (III) prepared in Example 1 in 200 μL of DMF, add 3mmoL of DCC and 3mmoL of NHS, and activate by shaking at 4°C for 12 hours in the dark to obtain an activated malathion derivative ;
[0117] 1.2 Centrifuge the malathion derivative described in step 1.1 at 12,000 rpm for 10 min, collect the supernatant, and obtain the malathion hapten derivative. Prepare a solution containing 5 mg / mL carrier protein BSA with a PBS buffer solution with a pH of 7.4;
[0118] 1.3 Add the hapten derivatives described in step 1.2 dropwise to the PBS buffer solution containing the carrier protein, so that the molar ratio of the malathion hapten to the carrier protein is 400:1, shake and react at 4°C for 10 h, That is to obtain the malathion artificial antige...
Embodiment 3
[0123] Example 3 Preparation of Malathion Polyclonal Antibody
[0124] 1. Experimental steps
[0125] Preparation of malathion polyclonal antibody, specific steps include:
[0126] 1.1 Mix and emulsify the prepared malathion artificial antigen MLH-BSA with an equal amount of complete adjuvant as an immunogen, select mice as experimental animals, and inject subcutaneously into the abdomen, back, and soles of each mouse at a rate of 50 μg ( Calculated by protein content) dose to immunize 6-week-old mice for initial immunization;
[0127] 1.2 The second immunization: Three weeks later, replace the complete adjuvant with incomplete adjuvant, and boost the immunization once, and the operation method is the same as step 1.1;
[0128] 1.3 The third booster immunization: booster immunization once every two weeks, the operation method is the same as step 1.2;
[0129] 1.4 Blood collection: blood was taken from the tail, and after standing at 37°C for 30 minutes, it was centrifuged a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com