Expression preparation and application of porcine circovirus type 2 recombinant Cap protein
A porcine circovirus and recombinant protein technology, applied in the biological field, can solve problems such as uneven levels of subunit vaccines, unsatisfactory immune effects, and difficult inactivation effects of inactivated vaccines, achieving good biological activity and immunogenicity, The effect of high VLP assembly rate and reduction of production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
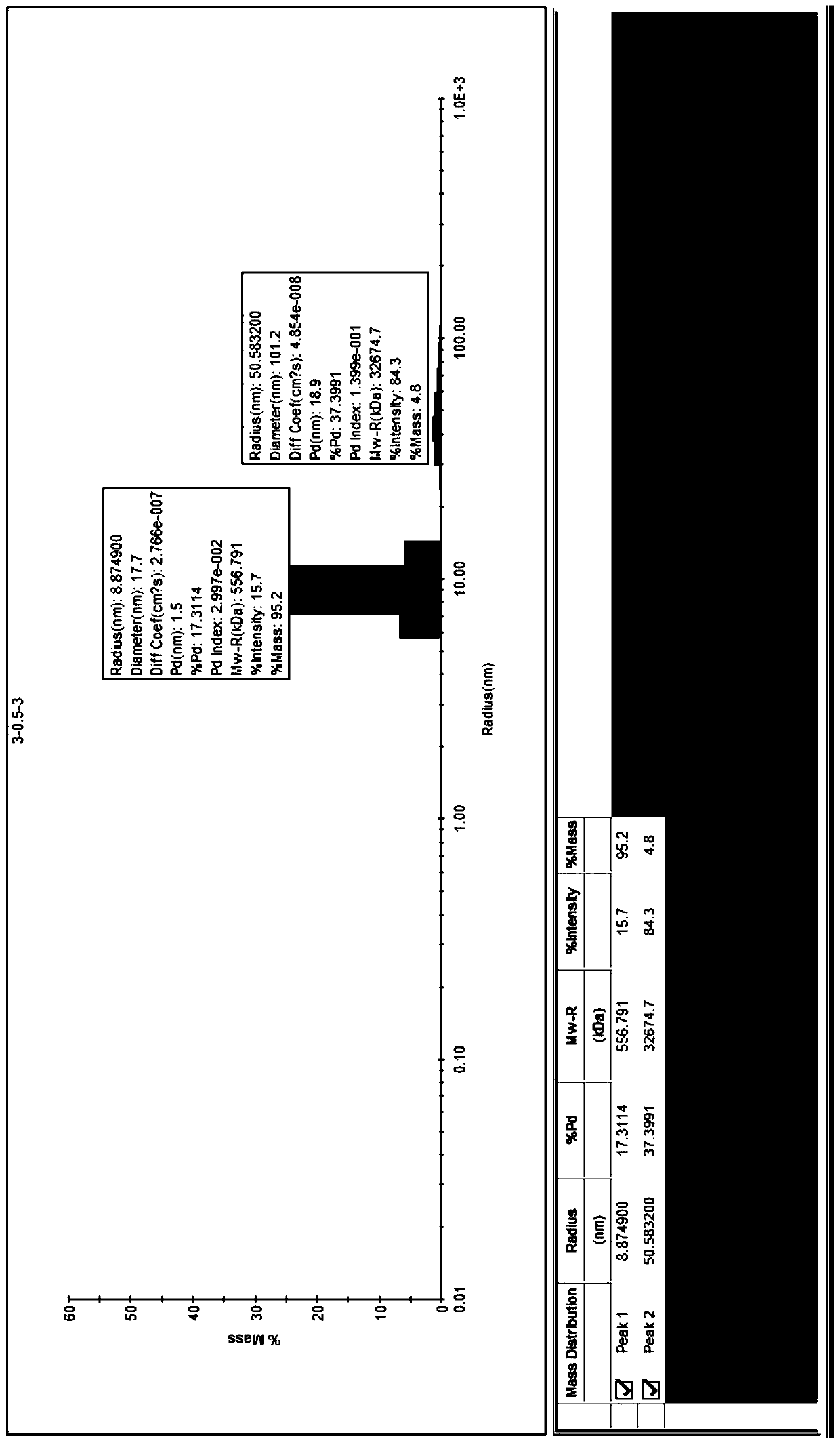
Image
Examples
Embodiment 1
[0033] Embodiment 1, the preparation of porcine circovirus type 2 recombinant Cap protein
[0034] 1. Experimental materials
[0035] The primers were synthesized from Shanghai Jierui Bioengineering Co., Ltd., and the competent E. coli BL21 (DE3) was preserved in the laboratory;
[0036] Main reagents DNA plasmid small extraction kit; peptone and yeast extract were purchased from Oxoid; IPTG, L-arginine hydrochloride, Tris-HCL were purchased from Amresco; BSA protein standard was purchased from Thermo Fisher Scientific;
[0037] 2. Construction of porcine circovirus type 2 recombinant Cap protein expression plasmid
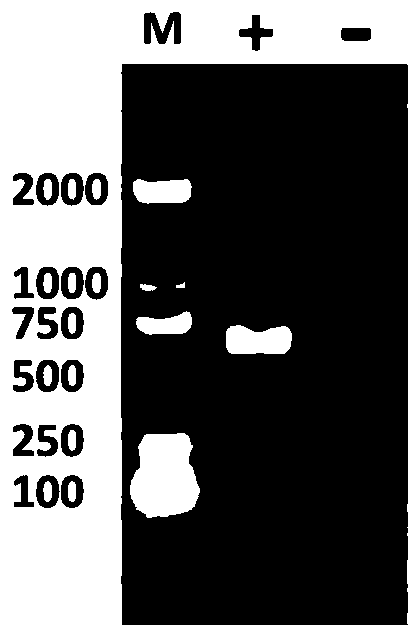
[0038]The recombinant Cap gene sequence comes from the prevailing strain of PCV2 isolated in our laboratory. The recombinant Cap gene sequence is analyzed and compared, and the structure of the Cap protein sequence is predicted after removing 41 basic amino acids at the N-terminus, and compared with the resolved PCV2Cap protein structure. (PDB: 3R0R) for structu...
Embodiment 2
[0062] The preparation of embodiment 2 porcine circovirus type 2 recombinant Cap protein subunit vaccines
[0063] 1. Protein preparation
[0064] Pick pET-CapR and transform Escherichia coli BL21 (DE3) competently to obtain a single colony of recombinant bacteria expressing recombinant Cap protein E.coli-PCV2-FJ-Cap to prepare recombinant Cap protein according to the method of steps 3-7 in Example 1, namely : Escherichia coli E.coli-PCV2-FJ-Cap CGMCCNo.18235 was inoculated in 20ml LB medium, cultivated overnight at 37°C and 200rpm as the seed solution, and the next day the seeds were mixed at a ratio of 1:100 Inoculate 5L of LB medium containing Kanna resistance, culture at 37°C, 200rpm for 2-3h, when the OD600 of the bacterial solution reaches 0.6-0.8, add IPTG with a final concentration of 1mmol / L, and continue to induce culture at 37°C for 5h. The bacteria were centrifuged at 5000rpm for 10min to collect the bacteria, and the bacteria were resuspended in 500mL PBS, and th...
Embodiment 3
[0070]Example 3: Immunogenicity Research of Porcine Circovirus Type 2 Recombinant Cap Protein Subunit Vaccine
[0071] 1. Piglet safety test
[0072] 35 circoantigen-antibody double-negative piglets aged 2-3 weeks were screened for the safety test of circovirus type 2 recombinant Cap protein subunit vaccine. Grouping situation: 30 piglets were randomly selected and divided into 5 groups, 5 piglets per group, as the vaccine immunization group, each pig in immunization group 1 was immunized with 4ml of circovirus type 2 recombinant Cap protein subunit vaccine containing 5 μg / ml recombinant Cap protein In immunization group 2, each pig was immunized with circovirus type 2 recombinant Cap protein subunit vaccine 4ml containing 10 μg / ml recombinant Cap protein; in immunization group 3, each pig was immunized with circovirus type 2 recombinant vaccine containing 20 μg / ml recombinant Cap protein Cap protein subunit vaccine 4ml, each pig in immunization group 4 was immunized with cir...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Radius | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com