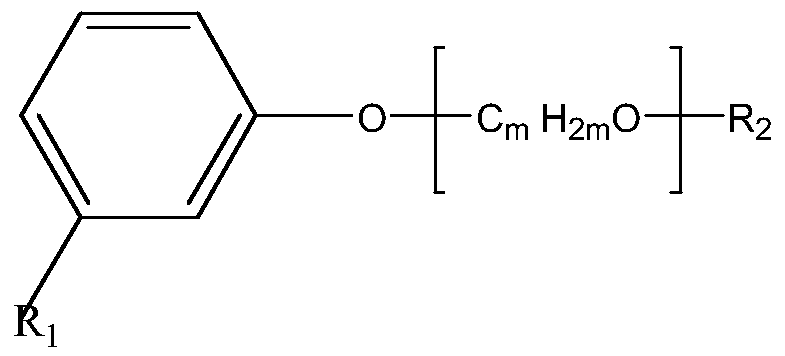
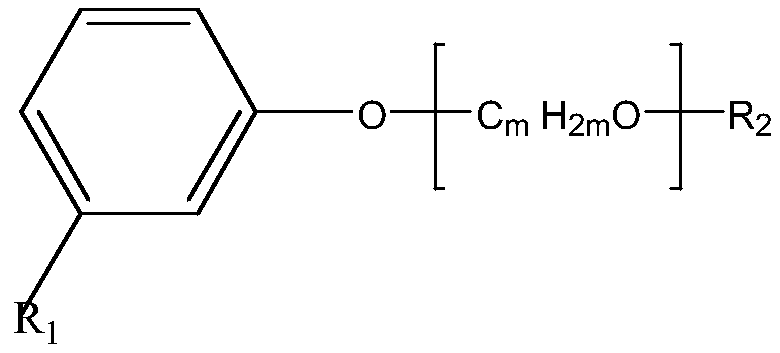
Amino-terminated polyether compound containing cardanol derivative, as well as preparation method and application of amino-terminated polyether compound
An amine-terminated polyether and compound technology, which is applied in the field of polyetheramine compound synthesis, can solve the problems of high technical difficulty, high cost, limited application of epoxy resin, etc., and achieves excellent toughness, high bonding strength, and improved interface properties. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Using 0.1 mol of polyethylene glycol (molecular weight 100) and 0.3 mol of thionyl chloride as raw materials, 1 ml of N,N-dimethylformamide as a catalyst, stirred and reacted at 60°C for 10 hours to obtain double-terminal chlorine-terminated Polyether compounds;
[0039] After mixing 0.1 mol of cardanol, 0.12 mol of sodium hydroxide solution and 1.5 g of tetrabutylammonium bromide (TBAB), add 0.1 mol of double-terminal chlorine-terminated polyether compound, and stir at 50 ° C for 12 hours. Then extract, filter to remove impurities, pass through carbon dioxide to wash with water, and distill off the solvent to obtain the chlorinated cardanol polyether compound;
[0040] After mixing 0.1 mol of chlorinated cardanol polyether compound, 100 grams of toluene, 0.5 mol of ethylenediamine solution and 1 g of tetrabutylammonium bromide (TBAB), stir at 70 ° C for 20 hours, then wash with water, separate liquid and the solvent was distilled off to obtain the amino-terminated pol...
Embodiment 2
[0042] Using 0.1mol of polyethylene glycol (molecular weight 1000) and 0.4mol of thionyl chloride as raw materials, 3ml of N,N-dimethylformamide as a catalyst, stirred and reacted at 70°C for 8 hours to obtain double-terminal chlorine-terminated Polyether compounds;
[0043] After mixing 0.1 mol of cardanol, 0.15 mol of sodium hydroxide solution and 2 g of tetrabutylammonium bromide (TBAB), add 0.1 mol of double-terminal chlorine-terminated polyether compound, stir at 60 ° C for 10 hours, and then Extracting, filtering to remove impurities, passing through carbon dioxide to wash with water, and distilling off the solvent to obtain the chlorinated cardanol polyether compound;
[0044] Mix 0.1mol of chlorinated cardanol polyether compound, 100 grams of toluene, 0.4mol of diethylenetriamine, solution and 2g of tetrabutylammonium bromide (TBAB), stir at 80°C for 15 hours, and then wash with water , liquid separation and distillation to remove the solvent to obtain the amino-termi...
Embodiment 3
[0046] Using 0.1 mol of polyethylene glycol (molecular weight 6000) and 0.7 mol of thionyl chloride as raw materials, 5 ml of N,N-dimethylformamide as a catalyst, stirred and reacted at 80°C for 4 hours to obtain double-terminal chlorine-terminated Polyether compounds;
[0047] After mixing 0.1 mol of cardanol, 0.2 mol of sodium hydroxide solution and 3.0 g of tetrabutylammonium bromide (TBAB), add 0.1 mol of double-terminal chlorine-terminated polyether compound, and stir at 80 ° C for 6 hours. Then extract, filter to remove impurities, pass through carbon dioxide to wash with water, and distill off the solvent to obtain the chlorinated cardanol polyether compound;
[0048] After mixing 0.1mol of chlorinated cardanol polyether compound, 100 grams of toluene, 0.15mol of propylenediamine solution and 3g of tetrabutylammonium bromide (TBAB), stir at 90°C for 10 hours, then wash with water, separate liquid and the solvent was distilled off to obtain the amino-terminated polyethe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com