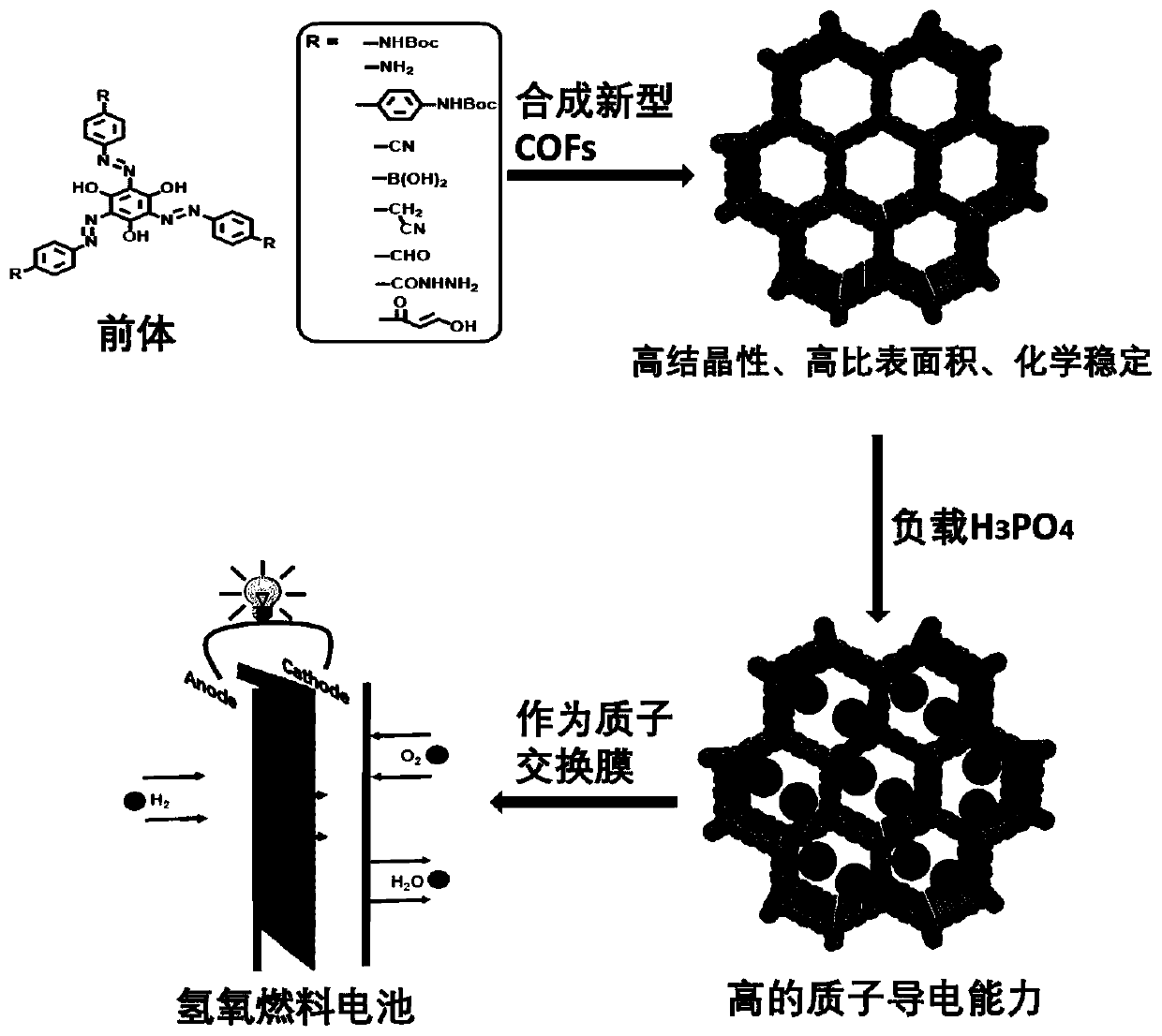
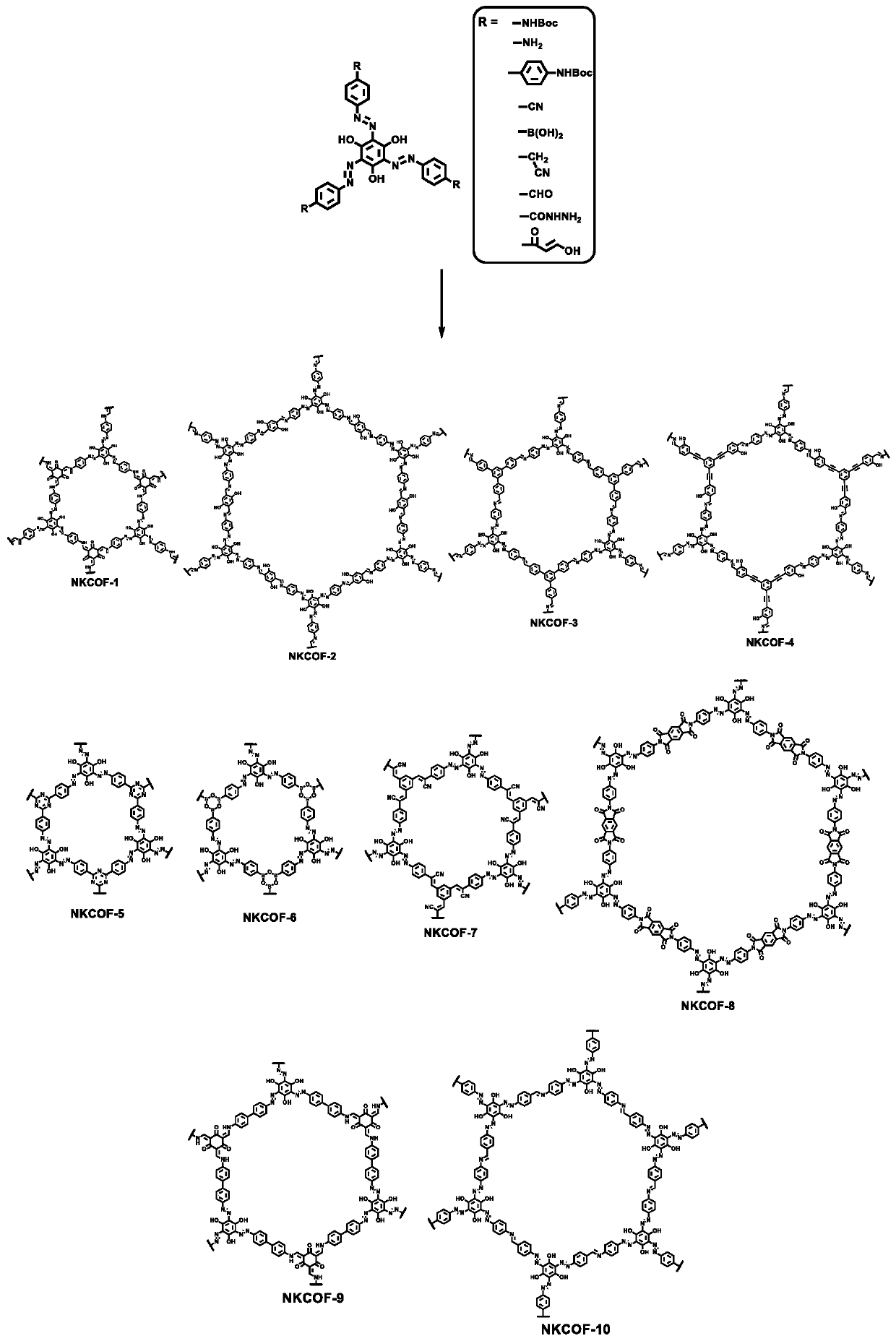
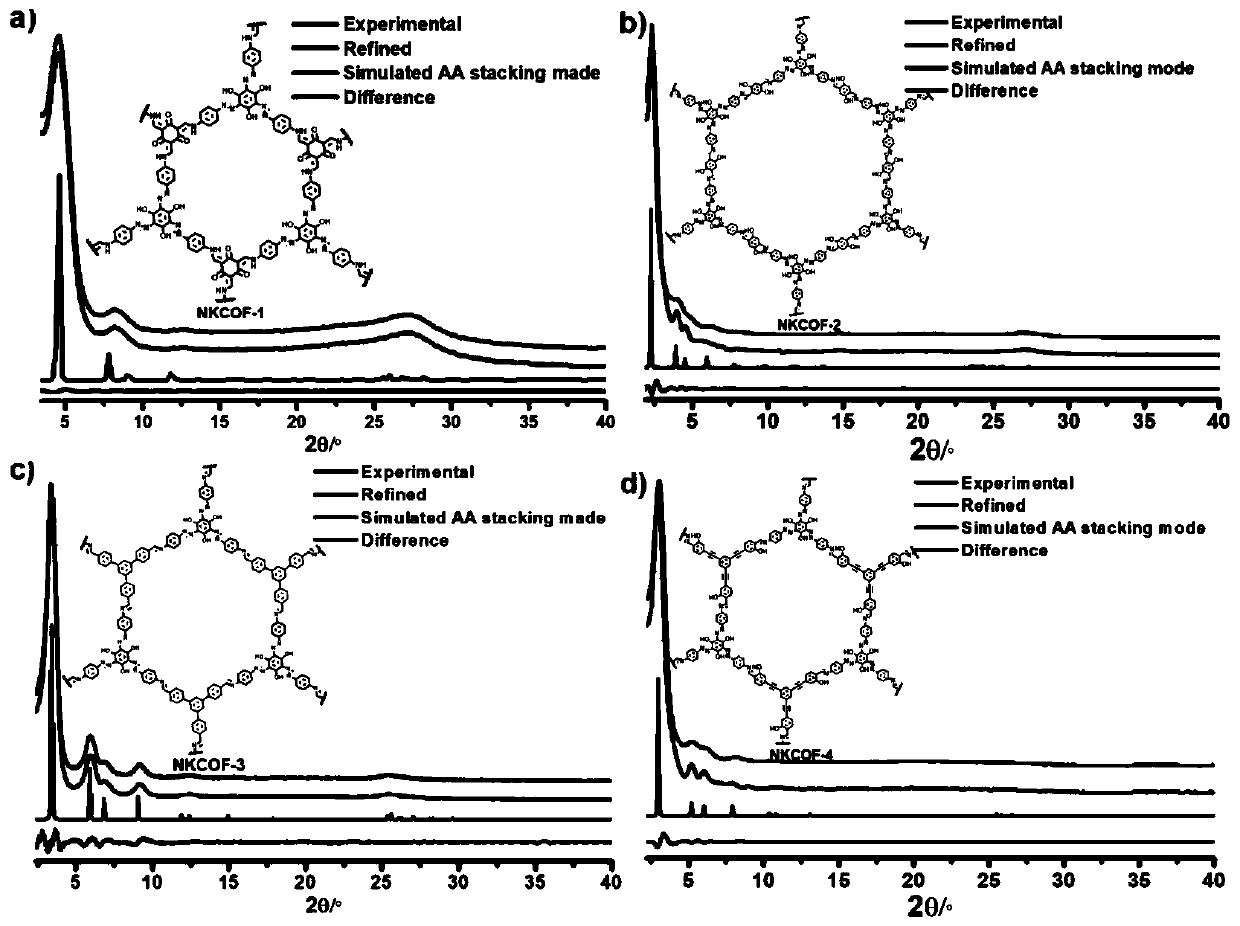
Preparation method of azo bond-rich covalent organic framework materials and application of azo bond-rich covalent organic framework materials in proton conduction and fuel cells
A covalent organic framework and aromatic azo technology, applied in fuel cells, circuits, electrical components, etc., can solve the problem of low proton conductivity and achieve the effects of cheap raw materials, mild reaction conditions, and simple experimental operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The synthesis of a series of organic small molecule precursors rich in hydroxyl and azo groups, the specific implementation steps are as follows:
[0036] The new small molecular precursors containing high-density azo bonds mainly use the bond-forming reaction of azo. Contains amino-NH 2 The monomer (10 mmol) was dissolved in 20 mL of 1 molar hydrochloric acid, and NaNO was slowly added in an ice-water bath 2 (11mmol), after the addition, keep stirring in an ice-water bath for 1 hour. Next, trisphenol (3 mmol) was dissolved in 20.2 mL of 1M NaOH aqueous solution, and cooled to 0-5°C. Then it was slowly added dropwise into the previous reaction system, and after the dropwise addition was completed, the reaction was continued for 4 hours. A large amount of red-black solid was produced, filtered and dried to obtain the product (80% yield).
[0037] The synthesis of a series of new COFs, the specific implementation steps are as follows:
[0038] The synthesis of NKCOFs...
Embodiment 2
[0045] Example 2: Loading of NKCOFs phosphoric acid
[0046] 60 mg of NKCOFs powder was soaked in 5 M phosphoric acid aqueous solution for 12 hours, filtered, and then the solid was washed thoroughly with water until the eluent reached pH = 7, and then the resulting sample was dried at 120 °C for 24 hours to obtain dried phosphoric acid-loaded NKCOFs ( named H 3 PO 4 @NKCOFs). Selection of soaking time: Experiments have found that the soaking time is in the range of 0 to 12 hours. The longer the soaking time, the higher the proton conductivity. When the soaking time exceeds 12 hours, the proton conductivity will no longer increase. Thus choose 12 hours as the preparation H 3 PO 4 Soak time for @NKCOFs. Calculated phosphoric acid in H by thermogravimetry and other tests 3 PO 4 Final load in @NKCOFs.
Embodiment 3
[0047] Embodiment 3: the preparation of NKCOF film
[0048] NKCOF membranes can be prepared by powder compression and direct synthesis. Powder compression: take 50 mg of NKCOFs powder, and use an infrared mold to press it into a circular sheet with a diameter of 1.3 cm and a thickness of 200-400 μm under a pressure of 20 MPa. Direct synthesis: mix a certain amount of Azo-NH2, aldehyde, p-toluenesulfonic acid and water, grind it into a uniformly dispersed paste in a mortar, and then spread the paste evenly on a clean glass plate , reacted at 90 °C for 24 hours, washed with hot water and dried to obtain a crystalline, self-supporting COF membrane. In addition, the membrane can also be obtained by suction filtration after stripping, or the COF monomer can be dissolved in different solvents, such as water phase and oil phase, through the interfacial method, and a high-quality COF membrane can be obtained through interfacial diffusion.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com