Hydrogen oxidation reaction catalyst for alkaline medium and synthesis method thereof
A technology of oxidation reaction and alkaline medium, applied in the direction of nanotechnology, structural parts, electrical components, etc. for materials and surface science, can solve the problems of low activity and expensive Pt-based catalysts, and achieve the goal of improving electrocatalytic performance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
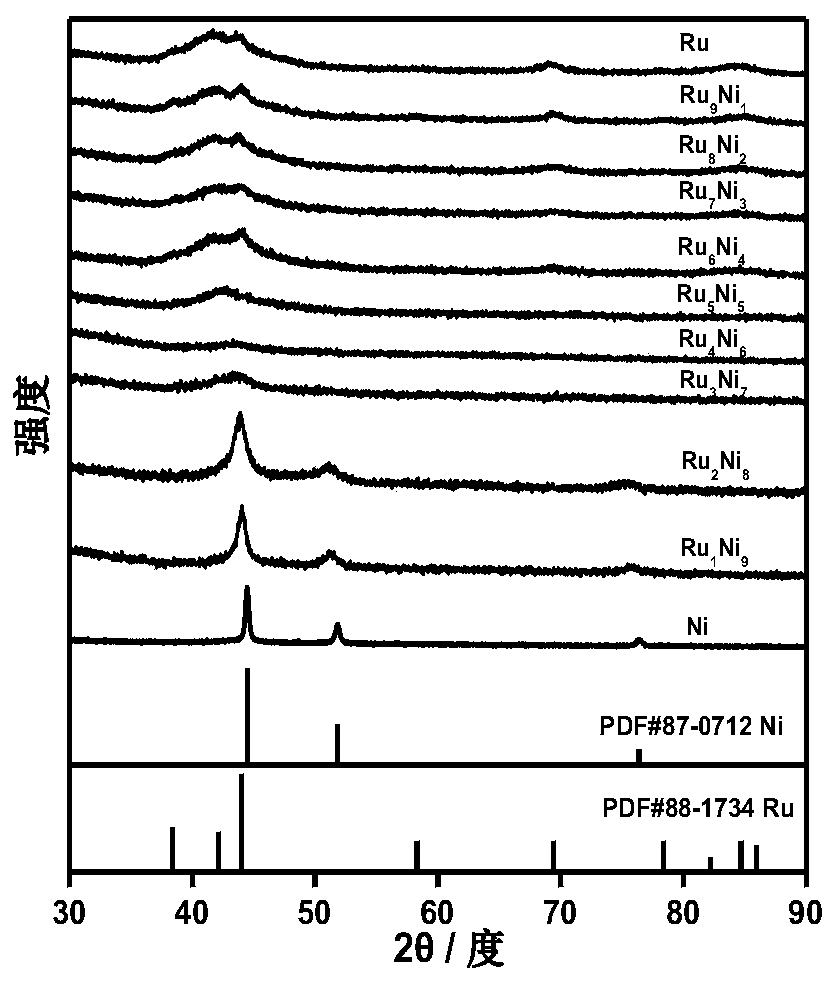
[0046] The invention provides a method for synthesizing a supported nanometer metal catalyst for hydrogen oxidation in an alkaline medium, comprising the following steps:
[0047] Dissolving the ruthenium source and the nickel source into oleylamine and toluene to form a first precursor solution;
[0048] adding a reducing agent to the first precursor to form a uniform first reaction solution;
[0049] heating the uniform first reaction solution to the first reaction temperature and maintaining it for a period of time to form a second reaction solution;
[0050] Separating and purifying the above second reaction solution to obtain bimetallic nanoparticles.
[0051] In some embodiments, the ruthenium source is ruthenium acetylacetonate, and the nickel source is nickel acetylacetonate.
[0052]In some embodiments, the first precursor solution, the first reaction solution, and the second reaction solution are all formed in a closed environment.
[0053] In some embodiments, th...
Embodiment 1
[0067] Bimetallic nanoparticles with a ruthenium-nickel ratio of 1:9 (Ru 1 Ni 9 / C) preparation method, experimental procedure is as follows:
[0068] 1. Take 3.18mg Ru(acac) 3 and 18.51mg Ni(acac) 2 The precursor was dissolved in a mixed solution of 6mL oleylamine and 2mL toluene, then the mixed solution was stirred and ultrasonicated for 30min, 25.06mg of p-dimethylaminobenzaldehyde was added, and then stirred and ultrasonically continued for 30min, the mixed solution was poured into 10mL of high temperature resistant poly Put the inner liner in a tetrafluoroethylene liner, then put the liner into a stainless steel reactor, and finally put the reactor into an oven, and react at 200°C for 12 hours.
[0069] 2. After the reaction in step 1 is over, pour the reaction solvent containing nanoparticles obtained in the liner into the centrifuge tube, then add absolute ethanol to wash, centrifuge at 11000rpm for 5min, and then pour off the supernatant , the process was repeated ...
Embodiment 2
[0073] Bimetallic nanoparticles with a ruthenium-nickel ratio of 5:5 (Ru 5 Ni 5 / C) preparation method, experimental procedure is as follows:
[0074] 1. Take 16mg Ru(acac) 3 and 10.28mg Ni(acac) 2 Dissolve in 6mL oleylamine and 2mL toluene solution, stir the mixed solution for 30min, then add 30mg p-dimethylaminobenzaldehyde, then continue stirring and ultrasonic for 30min, then add the mixed solution into 10mL high temperature resistant polytetrafluoroethylene liner , and then put the liner into a stainless steel reactor, and finally put the reactor into an oven, and react at 220° C. for 12 hours.
[0075] 2. The reaction solvent containing nanoparticles obtained in step 1 was poured into 50 mL of absolute ethanol solution, and then centrifuged with a centrifuge at a speed of 11000 rpm for 5 min. This process was repeated three times. Finally the nanoparticles were redispersed in 50 mL cyclohexane.
[0076] 3. Take by weighing 14.9mg active carbon powder (Vulcan XC-72),...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com