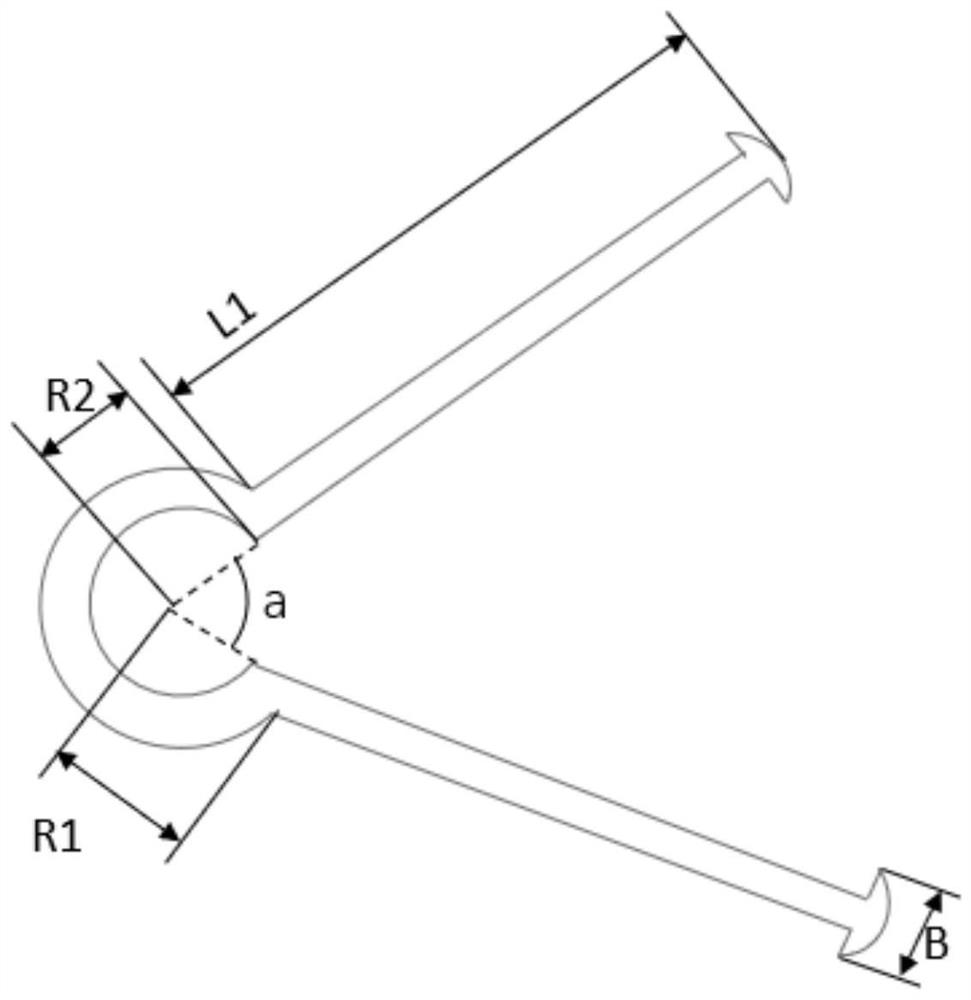
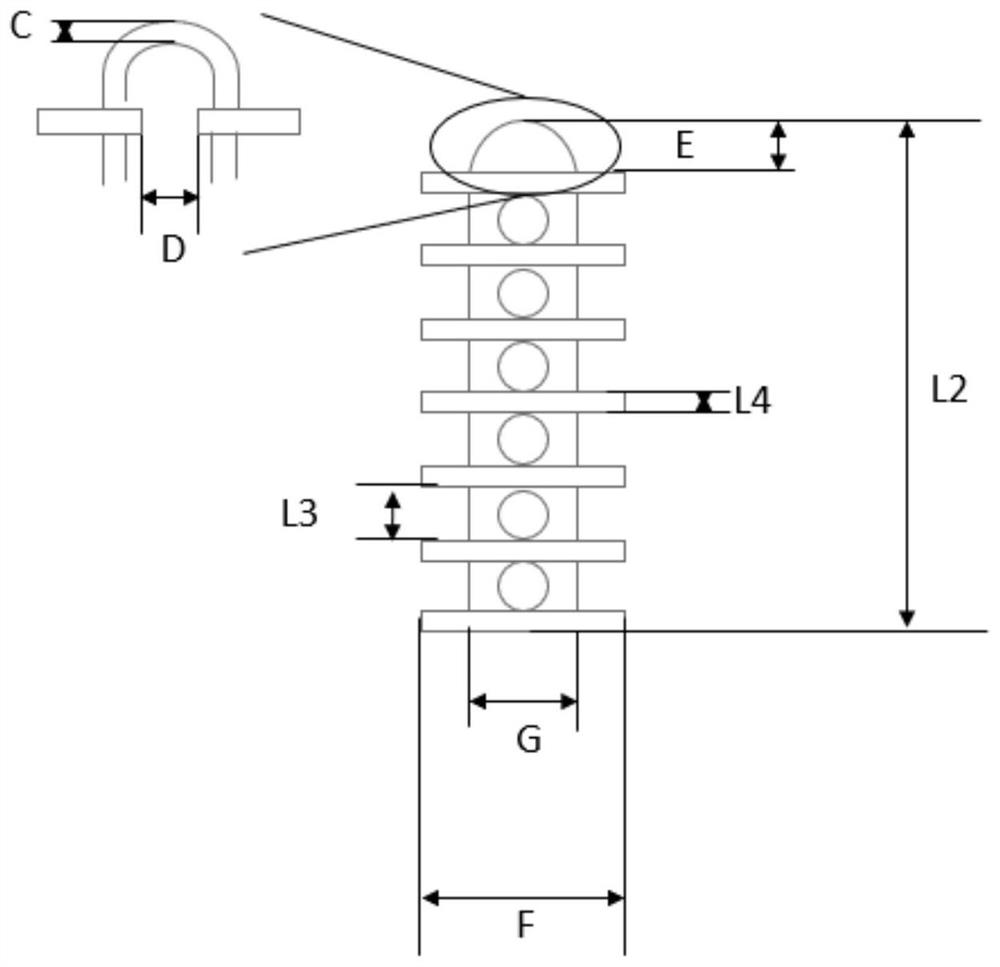
Device and method for controlling follicular development and preventing reproductive tract inflammation of equine animals
A follicle development and reproductive tract technology, applied in drug delivery, drug combination, pharmaceutical formulations, etc., can solve animal disease treatment and human health threats, drug-resistant bacteria, reduce the use of antibiotics, etc., to achieve auxiliary fixation of embolus, Less stress response and larger contact area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment one: female donkey is in estrus at the same time
[0060] In the National Donkey Breeding Center in Dong'e County, Shandong Province, 19 Dezhou black female donkeys aged 7-8 years old with normal fertility were selected and injected on the 0th day from April 15, 2018 to May 1, 2018 GnRH (1ml), on the 2nd day, the device of the present invention was put into the female donkey's vagina (CRID), and on the 8th day, prostaglandin (PG) 4ml was injected, and on the 11th day, the device of the present invention was taken out and injected with PGF4ml. From the 12th day to the 16th day, B-ultrasound examination was carried out every day to observe the follicular development of the female donkey and whether the device of the present invention could inhibit ovulation. When the B-ultrasound examination found that the follicle was greater than 32mm, it could be considered as estrus. Project process such as image 3 , the estrus results of female donkeys after implementati...
Embodiment 2
[0063] Embodiment two: female donkey estrus synchronously and artificial insemination
[0064] In the Dong’e Black Donkey Breeding Base in Aohan Banner, Inner Mongolia, 80 Dezhou black female donkeys with normal fertility and aged 7-8 were selected and injected with GnRH (1ml) on the 0th day from May to August 2018 On the 2nd day, insert the device of the present invention into the vagina of the donkey, inject 4ml of PG on the 8th day, take out the device of the present invention and inject 4ml of PG on the 11th day, and the donkey will perform B-ultrasound every day from the 12th to the 16th day Check to observe the follicle development of the donkey and whether the device of the present invention can inhibit ovulation. When the B-ultrasound examination finds that the follicle is greater than 32mm, it can be considered as estrus. After 12 hours, artificial insemination was carried out with fresh semen, the volume of each insemination was 10ml, and the total effective sperm co...
Embodiment 3
[0067] Embodiment 3: Simultaneous estrus and artificial insemination of female donkeys
[0068] At Zhangjiakou Sangyang Animal Husbandry Co., Ltd. in Hebei Province, 76 Dezhou black female donkeys with normal fertility and aged 4-8 years were selected and divided into two batches from July to August 2019. GnRH (1ml) was injected on the 0th day, the device of the present invention was inserted into the donkey's vagina on the 2nd day, PG 4ml was injected on the 8th day, the device of the present invention was taken out on the 11th day and PG 4ml was injected, and the donkey arrived on the 12th day. On the 16th day, B-ultrasound examination was carried out every day to observe the follicle development of the female donkey and whether the device of the present invention could inhibit ovulation. When the B-ultrasound examination found that the follicle was greater than 32mm, it could be considered as estrus. After 12 hours, artificial insemination was carried out with fresh semen, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com