Hoof nail polypeptide tablet and preparation process thereof
A preparation process and technology of hoof nails, applied in the field of hoof nail polypeptide tablets and its preparation, can solve the problems of inability to exert the hoof hoof polypeptide function, affect the hoof hoof polypeptide effect, uneven drug mixing, etc., and achieve the improvement of endometrial blood vessels Effects of blood flow disturbance, reduction of vascular permeability, and avoidance of adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
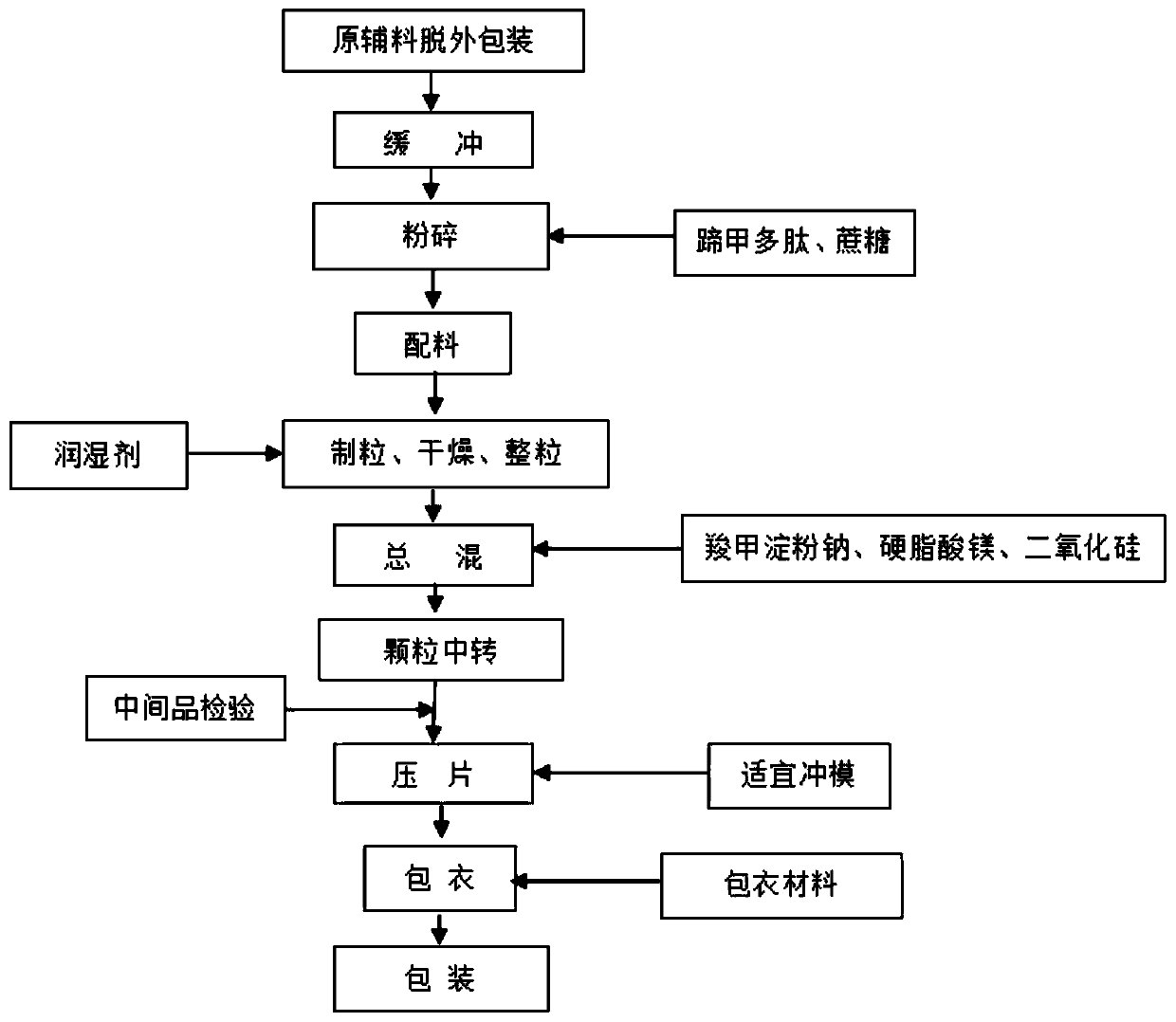
[0037] The invention provides a hoof nail polypeptide tablet and a preparation process thereof. The hoof nail polypeptide tablet consists of 35 parts of hoof nail polypeptide, 5 parts of corn starch, 15 parts of sucrose, 5 parts of carboxymethyl starch sodium, 0.2 parts of magnesium stearate, 0.5 parts of Part silicon dioxide, 8 parts of ethanol (60%) and 20 parts of ethanol (80%) are made, wherein when making coating, add 2.5 parts of pharmaceutical film coating premix.
[0038] Hoof nail polypeptide sheet preparation process such as figure 1 Shown:
[0039] Step 1, removing the outer packaging of the hoof nail polypeptide raw material;
[0040] Step 2, material preparation, prepare 35-40 parts of hoof nail polypeptide, 5-10 parts of corn starch, 15-20 parts of sucrose, 5-10 parts of carboxymethyl starch sodium, 0.2-0.4 parts of magnesium stearate, 0.5-0.8 parts of Silica, 8-12 parts of ethanol (60%) and 20-25 parts of ethanol (80%), add three quarters of water to 95% ethan...
Embodiment 2
[0057] The difference between embodiment two and embodiment one is only that the hoof nail polypeptide sheet is composed of 40 parts of hoof nail polypeptide, 10 parts of cornstarch, 20 parts of sucrose, 10 parts of carboxymethyl starch sodium, 0.4 part of magnesium stearate, 0.8 part of dioxide Silicon, 12 parts of ethanol (60%) and 25 parts of ethanol (80%), wherein 4.5 parts of pharmaceutical film coating premix are added when making the coating.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com