Preparation of near-infrared fluorescent probe with PTT effect and aggregation-induced emission enhancement effect
A technology of aggregation-induced luminescence and fluorescent probes, applied in luminescent materials, phototherapy, organic chemistry, etc., can solve problems that hinder the application of fluorescent dyes, and achieve good photothermal conversion performance, good photostability and photobleaching resistance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
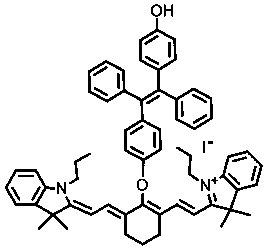
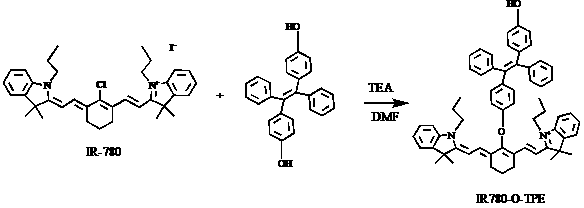
[0023] The synthesis of probe IR780-O-TPE, the synthetic route diagram is as follows figure 2 .
[0024] Concrete synthetic steps:
[0025] Add IR-780 iodide (20 mg, 0.03 mmol), 1,2-diphenyl-1,2-bis(4-hydroxybenzene)ethylene (40.0 mg 0.11 mmol) to a 10 mL two-necked bottle, vacuum-nitrogen Replace 3 times, then add 2 mL of anhydrous DMF and 0.2 mL of anhydrous triethylamine with a syringe. Under the protection of nitrogen, heat to 85 ° C for 5 h, TLC detection of the reaction, after the reaction is complete, without other treatment, the solvent is directly evaporated to dryness by the oil pump, the crude product is separated by a silica gel flash column, and CH 2 Cl 2 :CH 3 OH=20:1 eluent was eluted to obtain 20 mg dark green solid with a yield of about 80%. 1 H NMR (400 MHz, CDCl 3 ): δ 7.92 (d, 2H), 7.55(d, 2H), 7.35(t, 2H), 7.27–7.24 (m, 3H), 7.18 (t, 2H), 7.12−7.01 (m, 17H), 6.12 (d, 2H), 4.09 (t, 4H), 2.76 (t, 4H), 2.08 (t, 2H), 1.90-1.85 (m, 4H), 1.35 (s, 12H), 1...
Embodiment 2
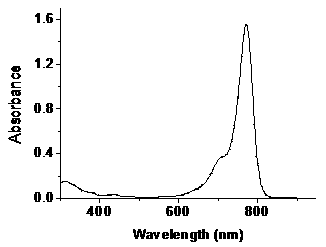
[0027] UV-visible absorption spectrum: prepare 10 μM ethanol solution of IR780-O-TPE and IR-780 iodide, and scan the UV-visible spectrum at 300–900 nm, the results are as follows image 3 .
Embodiment 3
[0029] AIEE property test: IR780-O-TPE is easily soluble in ethanol, but insoluble in n-hexane. Weigh an appropriate amount of IR780-O-TPE on a ten-thousandth analytical balance, add an appropriate amount of ethanol to dissolve, and make the concentration 1 mM as the mother solution. Use a pipette gun to draw 10 μL of the mother solution into the EP tube, add ethanol in proportion, and then, under vibration, add the solution into n-hexane to prepare n-hexane containing ƒ h 90%, 70%, 50%, 30%, 10%, and 0% ethanol / n-hexane mixed solution, so that the final concentration of the solution was 10 μM, and the prepared mixed solution was left at room temperature for 1 h, and the fluorescence spectrum was measured. Such as Figure 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com