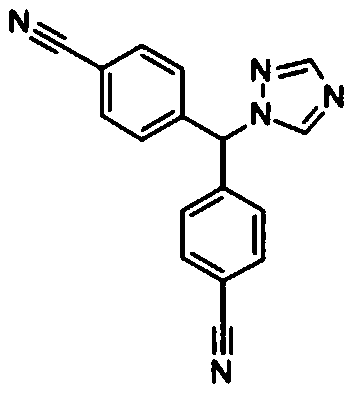
Letrozole tablet and preparation method thereof
A technology for letrozole tablets and tablet cores, which can be used in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
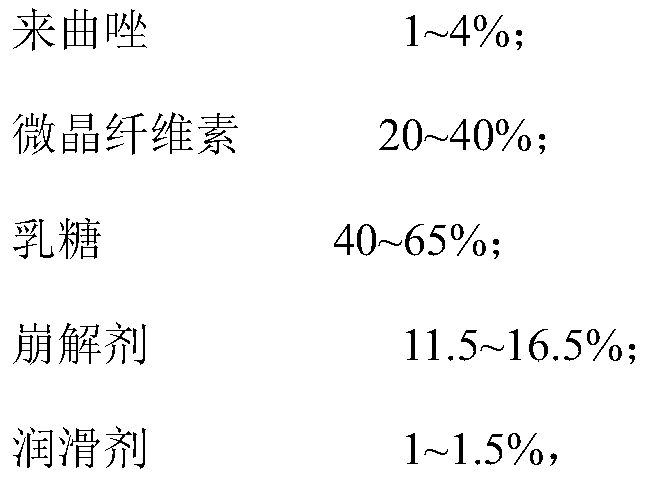
[0029] In order to further increase the uniformity of the mixing of the components, the above preparation method preferably includes: step S1, mixing the micronized silica gel with the first part of lactose to form a first mixture; step S2, mixing the first mixture of terazole, the second part of lactose and the first mixture to form The second mixture; step S3, mixing the third part of lactose with the second mixture to form a third mixture; step. S4, mixing the microcrystalline cellulose, disintegrant, the fourth part of lactose and the third mixture to form a fourth mixture; Step S5, mixing magnesium stearate with the fourth mixture to form a fifth mixture; and step S6, compressing the fifth mixture to obtain a tablet core.
[0030] In the above preparation method, the lactose is divided into four parts and added separately, and the micropowdered silica gel and the first part of lactose are mixed to remove the static electricity of the mixing equipment and reduce the adsorption...
Embodiment 1
[0041] Pretreatment
[0042] 1.760 kg of lactose (100 mesh) and 0.160 kg of silicon dioxide were jointly passed through a 40-mesh vortex vibrating screen once, and put into a container lined with a double-layer plastic bag as the first material.
[0043] 0.400 kg of letrozole and 1.520 kg of lactose (100 mesh) were passed through a 40-mesh vortex vibrating sieve 3 times, and put into a container lined with a double-layer plastic bag as the second material.
[0044] 3.840 kg of lactose (100 mesh) was passed through a 40-mesh vortex vibrating screen once and put into a container lined with a double-layer plastic bag as the third material.
[0045] Pass 2.720kg of lactose (100 mesh), 3.200kg of microcrystalline cellulose 102, 0.800kg of sodium carboxymethyl starch, 1.520kg of corn starch, together through a vortex vibrating sieve 40 mesh screen twice, and put them into a double-layer plastic bag lined In the container, as the fourth material.
Embodiment 2
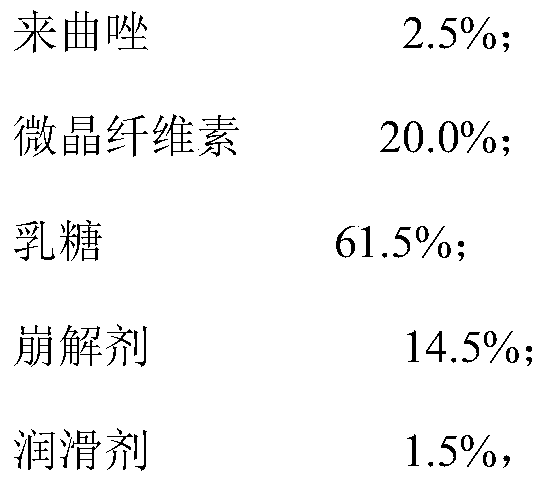
[0065] The amount of lactose in the first material was 1.968 kg, the amount of lactose in the second material was 1.476 kg, the amount of lactose in the third material was 3.936 kg, and the amount of lactose in the fourth material was 2.460 kg. The rest is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com