In-situ prepared nickel-based catalyst for catalyzing hydrogen absorption and release of liquid organic hydrogen carrier, and preparation method thereof
A nickel-based catalyst, in-situ preparation technology, applied in the field of hydrogen storage, can solve problems such as high cost of precious metal catalysts, and achieve the effect of excellent catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 12 mg of bis(1,5-cyclooctadienyl) nickel (Ni(COD) to the autoclave 2 ), 2.5 g N -Ethylcarbazole (NEC) and 250 mg γ-Al 2 O 3 After vacuuming and leak detection, heat to 80 ℃ for 2 h, then heat to 180 ℃, fill with 7 MPa hydrogen, and react for 2 h to obtain Ni / Al 2 O 3 catalyst.
[0025] Catalyze the catalyst directly in the reactor N -Ethylcarbazole (NEC) performance evaluation of hydrogen absorption and desorption, the hydrogen absorption reaction can be at 180 ℃, 1MPa H 2 It can be completed in 36 h under the conditions, and the corresponding hydrogen release reaction can be carried out at 200 ℃, 0.1MPa H 2 Under the conditions of 20 h, the hydrogen desorption exceeded 5.2 wt% (hydrogen yield 90%). The schematic diagram of the device for testing the hydrogen absorption and desorption kinetic curve of the liquid organic hydrogen carrier is shown in Figure 4 .
Embodiment 2
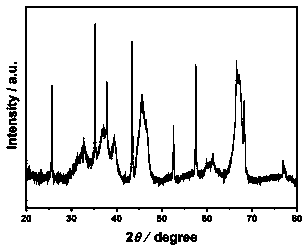
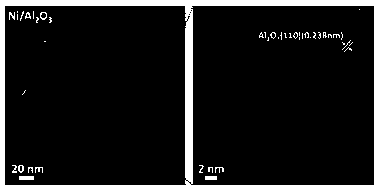
[0027] Add 24 mg of bis(1,5-cyclooctadienyl) nickel Ni(COD) to the autoclave 2 , 5 g N -Ethylcarbazole (NEC) and 250 mg γ-Al 2 O 3 After vacuuming and leak detection, heating to 80 ℃, holding for 2 h, then heating to 150 ℃, filling with 5MPa hydrogen, and reacting for 2 h to obtain Ni / Al 2 O 3 catalyst. The Ni / Al 2 O 3 The X-ray diffraction spectrum of the catalyst is shown in figure 1 , Ni / Al 2 O 3 See the transmission electron microscope photo figure 2 .
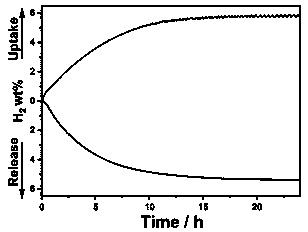
[0028] Catalyze the catalyst directly in the reactor N -Ethylcarbazole (NEC) performance evaluation of hydrogen absorption and desorption, the catalyst Ni / Al 2 O 3 catalytic N -Ethylcarbazole kinetic curve of hydrogen absorption and desorption see image 3 . The hydrogen absorption reaction can be at 180 ℃, 7 MPaH 2 It can be completed in 20 h under the conditions, and the corresponding hydrogen release reaction can be performed at 200 ℃, 0.1 MPa H 2 Under the conditions of 20 h, hydrogen desorption exceeded 5.6 wt% (hydrogen...
Embodiment 3
[0030] Add 24 mg of bis(1,5-cyclooctadienyl) nickel Ni(COD) to the autoclave 2 , 2.5 g N -Ethylcarbazole (NEC) and 250 mg of reduced graphene (rGO), vacuum and leak detection, heat to 80 ℃, hold for 2 h, then heat to 120 ℃, fill with 7 MPa hydrogen, react for 2 h to obtain Ni / rGO catalyst.
[0031] Catalyze the catalyst directly in the reactor N -Ethylcarbazole (NEC) performance evaluation of hydrogen absorption and desorption, the hydrogen absorption reaction can be at 180 ℃, 7 MPa H 2 It can be completed in 20 h under the conditions, and the corresponding hydrogen release reaction can be performed at 200 ℃, 0.1 MPa H 2 Under the conditions of 8 h, the hydrogen desorption exceeds 5.5 wt% (hydrogen yield 95%). The device for testing the hydrogen absorption and desorption kinetic curve of the liquid organic hydrogen carrier is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com