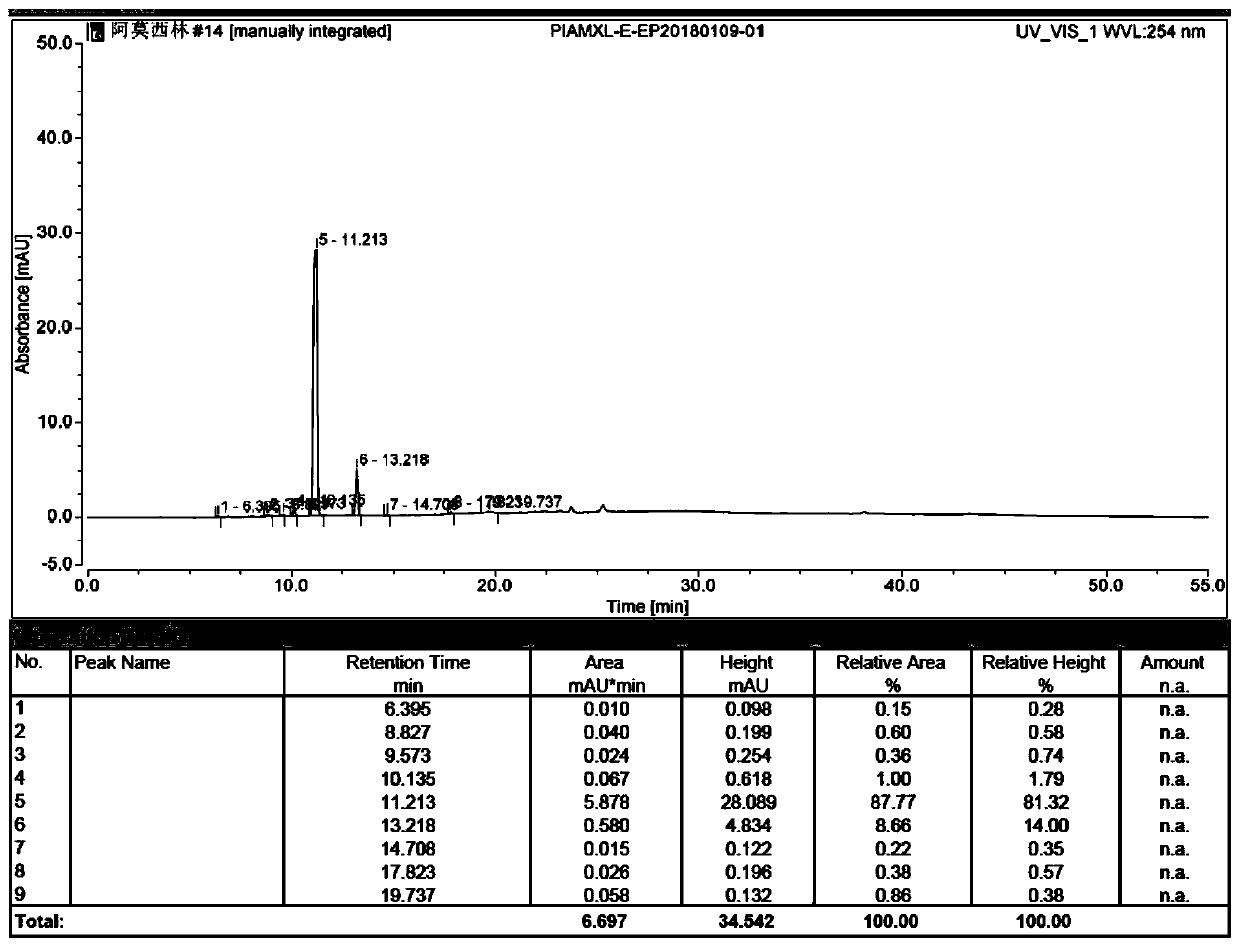
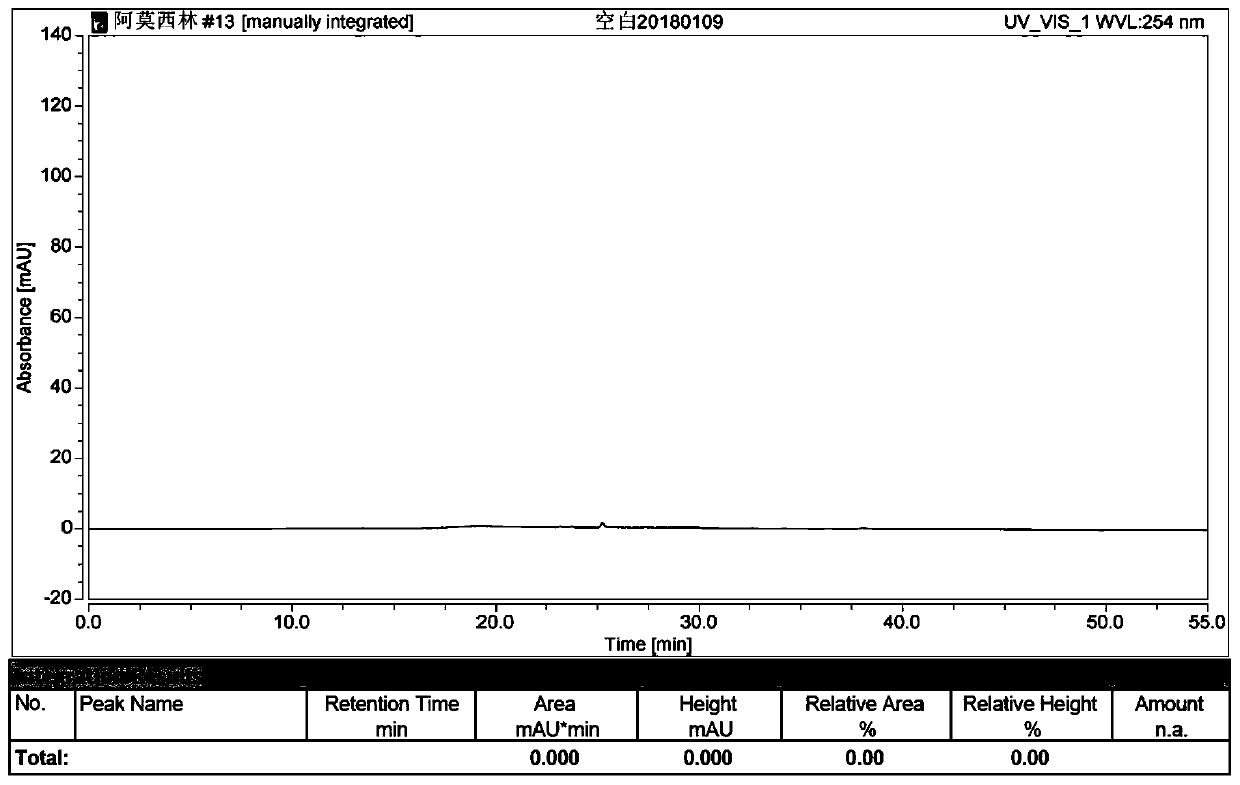
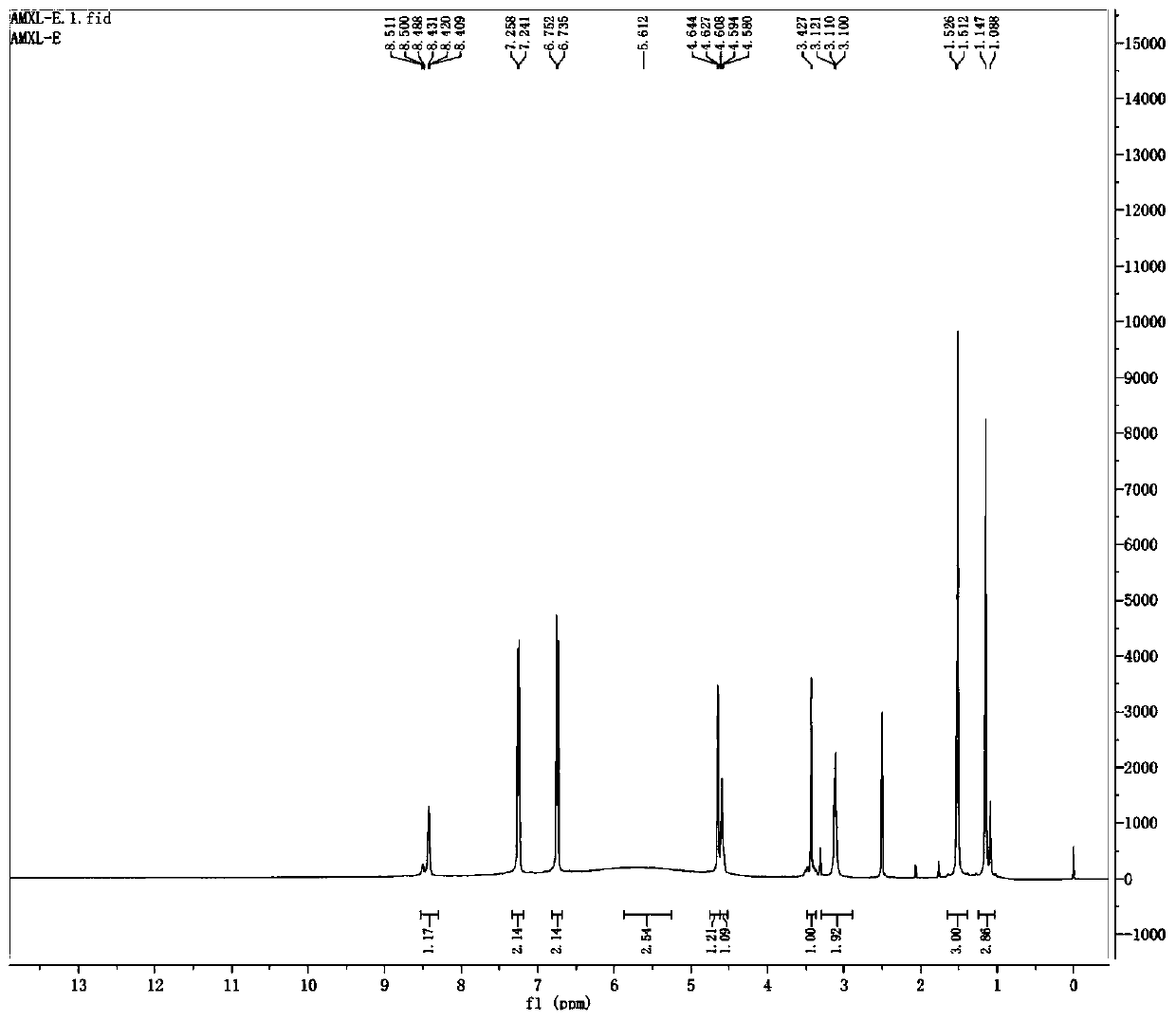
Preparation method of amoxicillin impurity E
An amoxicillin and impurity technology, applied in the field of medicinal chemistry, can solve the problems of affecting drug safety, difficult to obtain monomer impurities, carcinogenic teratogenicity of impurities, etc., to meet the needs of quality research, high purity and yield, and reaction conditions. mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A kind of embodiment of the preparation method of amoxicillin impurity E of the present invention, the preparation method of amoxicillin impurity E described in this embodiment is:
[0037] (1) Weigh 5g of amoxicillin, add 100mL of 0.5mol / L hydrochloric acid solution to the amoxicillin, heat and react at 50°C for 1h;
[0038] (2) adopting 1mol / L sodium hydroxide solution to adjust the pH value of the product obtained in step (1) to a pH value of 3.0 to obtain the amoxicillin impurity E reaction solution;
[0039] (3) Separation for the first time: Mix potassium dihydrogen phosphate aqueous solution and acetonitrile according to different volume ratios to obtain a plurality of mixed solutions; The second mixed solution taken off and the third mixed solution used for eluting for the third time; in the first mixed solution, the volume percentage of potassium dihydrogen phosphate aqueous solution is 97%, and the volume percentage of acetonitrile is 3%; the described In the...
Embodiment 2
[0042] A kind of embodiment of the preparation method of amoxicillin impurity E of the present invention, the preparation method of amoxicillin impurity E described in this embodiment is:
[0043] (1) Weigh 5g of amoxicillin, add 250mL of 0.3mol / L hydrochloric acid solution to the amoxicillin, and heat at 75°C for 1.5h;
[0044] (2) adopting 1mol / L sodium hydroxide solution to adjust the pH value of the product obtained in step (1) to a pH value of 4.0 to obtain the amoxicillin impurity E reaction solution;
[0045] (3) Separation for the first time: Mix potassium dihydrogen phosphate aqueous solution and acetonitrile according to different volume ratios to obtain a plurality of mixed solutions; The second mixed solution taken off and the third mixed solution used for eluting for the third time; in the first mixed solution, the volume percentage of potassium dihydrogen phosphate aqueous solution is 96%, and the volume percentage of acetonitrile is 4%; the described In the sec...
Embodiment 3
[0048] A kind of embodiment of the preparation method of amoxicillin impurity E of the present invention, the preparation method of amoxicillin impurity E described in this embodiment is:
[0049] (1) Weigh 5g of amoxicillin, add 500mL of 0.1mol / L hydrochloric acid solution to the amoxicillin, heat and react at 90°C for 2h;
[0050] (2) adopting 1mol / L sodium hydroxide solution to adjust the pH value of the product obtained in step (1) to a pH value of 5.0 to obtain the amoxicillin impurity E reaction solution;
[0051] (3) Separation for the first time: Mix potassium dihydrogen phosphate aqueous solution and acetonitrile according to different volume ratios to obtain a plurality of mixed solutions; The second mixed solution taken off and the third mixed solution used for eluting for the third time; in the first mixed solution, the volume percentage of potassium dihydrogen phosphate aqueous solution is 98%, and the volume percentage of acetonitrile is 2%; the described In the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| percent by volume | aaaaa | aaaaa |
| percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com