Preparation method of 2,9-dimethyl quinacridone purplish red pigment
A technology of dimethylquinacridone and dihydroquinacridone, which is applied in the field of preparation of 2,9-dimethylquinacridone purple pigment, can solve the problem of difficult removal and m-nitrobenzenesulfonate Toxicity and irritation, etc., to achieve the effect of reducing the cost of raw materials, reducing the cost of three wastes treatment, and reducing the amount of waste water produced
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
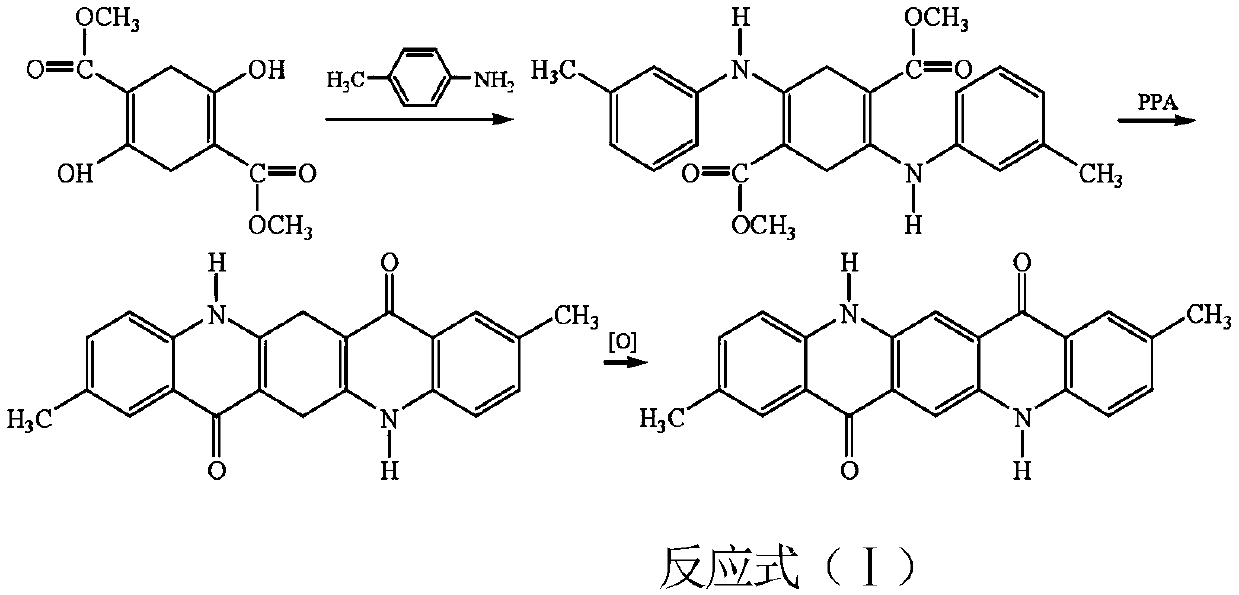
[0024] Ring-closing process: succinate diester self-condenses in the presence of sodium methoxide to obtain the first intermediate product dimethyl succinylsuccinate, and then adds aniline or substituted aniline to the reaction system for condensation reaction to obtain the second The intermediate product 2,5-dianiline-3,6-dihydroterephthalic acid dimethyl ester, which undergoes a ring-closure reaction under the action of polyphosphoric acid PPA, obtains the third intermediate product 2,9-dimethyl- 6,13-Dihydroquinacridone.
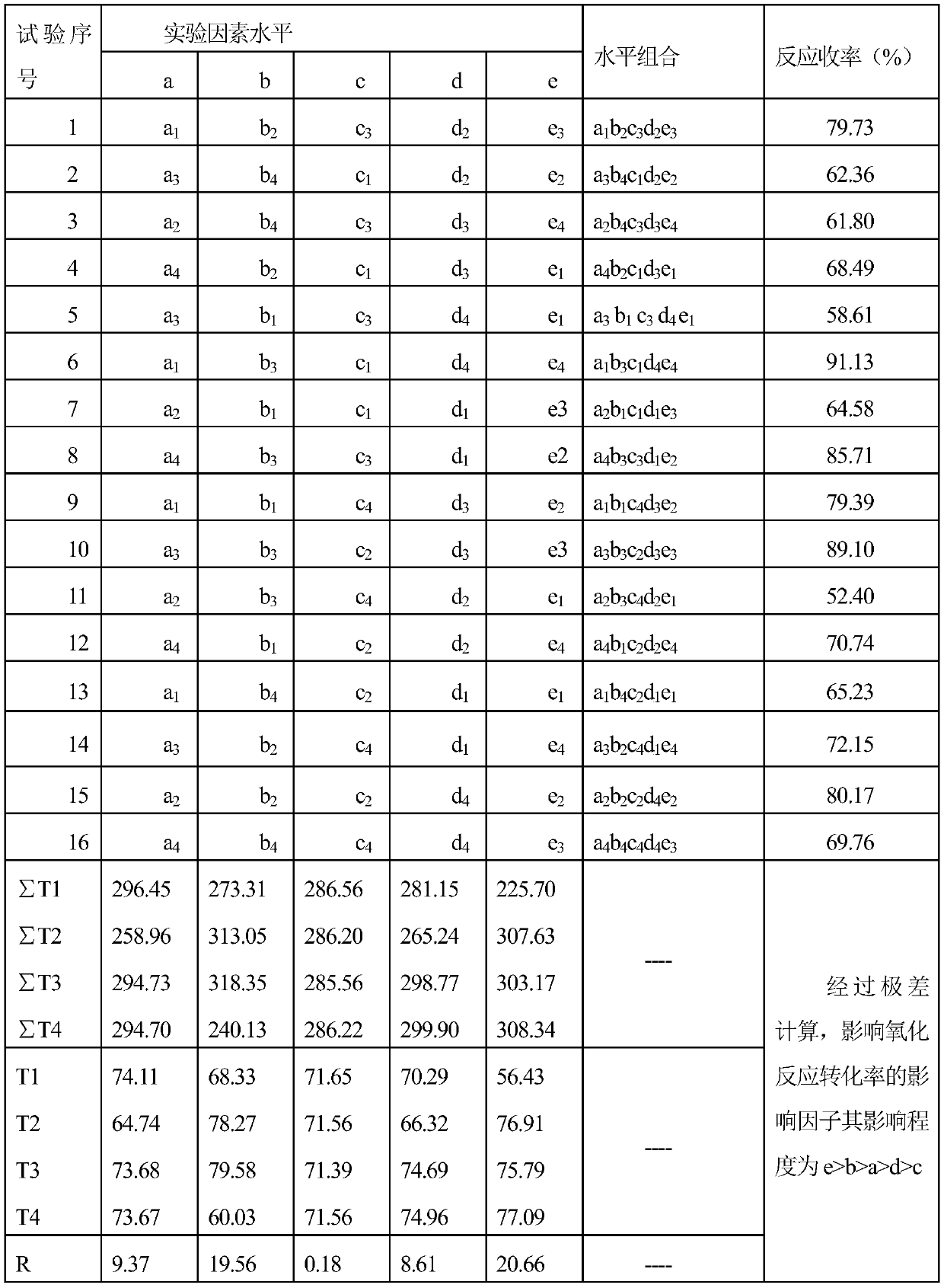
[0025] Oxidation process: Add 2,9-dimethyl-6,13-dihydroquinacridone obtained through condensation and ring-closing reaction into the reaction vessel, then add onionone-2-sodium sulfonate to it, and use KOH solution Adjust the pH to 10-11, heat up to 90°C, slowly add hydrogen peroxide solution into the reaction vessel, and control the hydrogen peroxide, allthone-2-sodium sulfonate and 2,9-dimethyl-6,13-dihydroquinacridine The molar ratio of pyridone added...
Embodiment 2
[0028] Oxidation process: Add 2,9-dimethyl-6,13-dihydroquinacridone obtained through condensation and ring-closing reaction into the reaction vessel, then add onionone-2-sodium sulfonate to it, and use KOH solution Adjust the pH to 7-8, heat up to 90°C, slowly add hydrogen peroxide solution into the reaction vessel, control The molar ratio of pyridone added is 1.3:0.07:1, continue to heat up to 130-140°C, keep the temperature, react for 4h, and the reaction ends; cool down the reaction solution to 80°C, pour it into a beaker, add the same volume as the reaction solution cold water, stirred and cooled to room temperature, filtered, the filter cake was washed with methanol, beating for 1 hour, filtered and top washed to obtain the crude PR122.
[0029] The ring-closing process and purification process are the same as in Example 1. The yield of the obtained product pigment purple PR122 was 85.71%.
Embodiment 3
[0031] Oxidation process: Add 2,9-dimethyl-6,13-dihydroquinacridone obtained through condensation and ring-closing reaction into the reaction vessel, then add onionone-2-sodium sulfonate to it, and use KOH solution Adjust the pH to 9-10, heat up to 90°C, slowly add hydrogen peroxide solution into the reaction vessel, and control the hydrogen peroxide, sodium allium-2-sulfonate and 2,9-dimethyl-6,13-dihydroquinacridine The molar ratio of pyridone added is 1.6:0.06:1, continue to heat up to 120-130°C, keep the temperature, react for 4h, and the reaction ends; cool down the reaction solution to 80°C, pour it into a beaker, add the same volume as the reaction solution cold water, stirred and cooled to room temperature, filtered, the filter cake was washed with methanol, beating for 1 hour, filtered and top washed to obtain the crude PR122.
[0032] The ring-closing process and purification process are the same as in Example 1. The yield of the obtained product pigment purple PR12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com