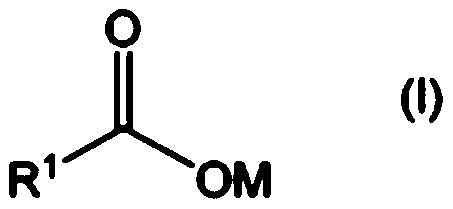
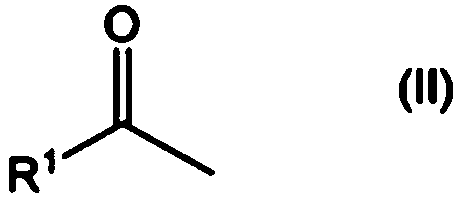
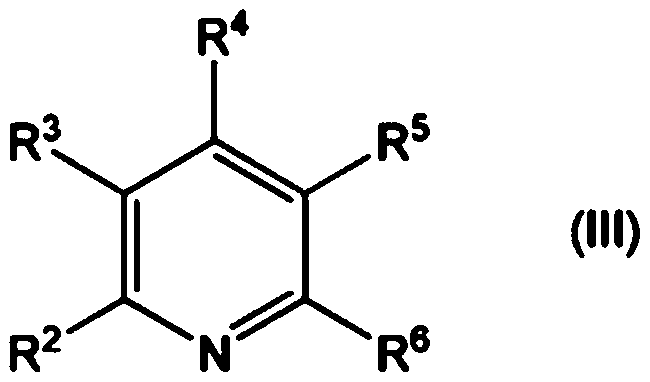
Method for producing aliphatic carboxylic acid compound and pyridine compound adduct of aliphatic ketone compound
A technology of aliphatic carboxylic acid and manufacturing method, which is applied in the directions of carboxylate preparation, carboxylate preparation, organic chemistry, etc., can solve problems such as unknown, and achieve the effect of safety and easy manufacture
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0164] Hereinafter, although an Example demonstrates this invention, this invention is not limited to these Examples. In addition, in Examples and Reference Examples, gas chromatography (gas chromatography, GC) was measured under the following conditions.
[0165]
[0166] Gas chromatograph: GC-2014 (Manufacturer: Shimadzu Corporation)
[0167] Column: DB-1ms (manufacturer: Agilent Technologies Inc., inner diameter: 0.25mm, film thickness: 0.25μm, length: 60m)
[0168] Carrier gas: helium, column flow rate 2.32mL / min
[0169] Injection conditions: 250°C, split ratio 30
[0170] Column temperature conditions: keep at 50°C for 5 minutes, then raise the temperature to 280°C at 20°C / min
[0171] Detector: Flame ionization detector (FID), 320°C
[0172]
[0173] (first step)
[0174] 11.7 g (0.1 mol) of diacetone alcohol and 30 mL of pyridine were put into a four-necked flask with a capacity of 500 mL, and mixed. 25.4 g (0.1 mol) of iodine was added to the obtained raw ma...
Embodiment 2
[0183] (first step)
[0184] 11.7 g (0.1 mol) of diacetone alcohol and 30 mL of pyridine were put into a four-necked flask with a capacity of 500 mL, and mixed. 25.4 g (0.1 mol) of iodine was added to the obtained raw material mixture, and it stirred for 90 minutes, heating in the water bath set to 60 degreeC, and obtained the reaction liquid. From the reaction liquid, unreacted pyridine was roughly removed by distillation under reduced pressure to obtain a reaction mixture containing a pyridine adduct. The reaction mixture was used in the next step without purification.
[0185] (second step)
[0186] To the reaction mixture containing the pyridine adduct was added an aqueous solution prepared by dissolving 8.4 g (0.2 mol) of sodium hydroxide in 100 mL of water, and stirred while heating in a water bath set at 90° C. for 1 hour to obtain a solution containing Hydrolyzate reaction mixture. The reaction mixture was used in the next step without purification.
[0187] (thir...
Embodiment 3
[0193] (first step)
[0194] 11.6 g (0.1 mol) of diacetone alcohol and 30 mL of pyridine were put into a four-necked flask with a capacity of 500 mL, and mixed. 25.6 g (0.1 mol) of iodine was added to the obtained raw material mixture, and it stirred for 90 minutes, heating in the water bath set to 60 degreeC, and obtained the reaction liquid. From the reaction liquid, unreacted pyridine was roughly removed by distillation under reduced pressure to obtain a reaction mixture containing a pyridine adduct. The reaction mixture was used in the next step without purification.
[0195] (second step)
[0196] To the reaction mixture containing the pyridine adduct was added an aqueous solution prepared by dissolving 10.0 g (0.25 mol) of sodium hydroxide in 100 mL of water, and stirred while heating in a water bath set at 90° C. for 1 hour to obtain a solution containing Hydrolyzate reaction mixture. The reaction mixture was used in the next step without purification.
[0197] (th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com