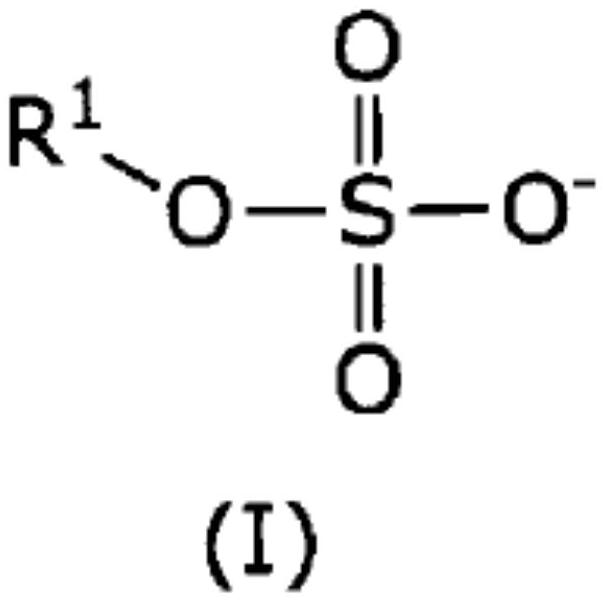Method for producing epoxy compound
A technology for epoxy compounds and manufacturing methods, applied in the direction of organic chemical methods, chemical instruments and methods, chemical/physical processes, etc., can solve the problems of reduced epoxy body selectivity, troublesome post-processing, etc., and achieve safe and simple manufacturing Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0141] Hereinafter, although an Example demonstrates this invention in more detail, this invention is not limited to these Examples.
[0142]
[0143] In the reaction container equipped with thermometer, stirrer, reflux tube, dropping device, drop into the diene compound 25.9g that formula (1) represents, toluene 6.58g, sodium tungstate 2 hydrate 1.42g, trioctyl methyl ammonium. 2.12 g of methylsulfate, 0.694 g of phenylphosphonic acid and 2.81 g of anhydrous sodium sulfate, after that, 32.7 g of 45% hydrogen peroxide was added dropwise over 4 hours while stirring at 25° C. hour response. The pH of the reaction solution during the reaction was 4.0 to 6.5. After the reaction, gas chromatography was used to analyze, and the conversion rate of the compound represented by the result formula (1) was 96%, and the selectivity of the epoxy compound represented by the formula (2) to formula (4) was 91% (yield 86%) , the selectivity of by-products was 9% (yield 8%).
[0144]
Embodiment 2
[0146] Put 25.9 g of diene compounds represented by formula (1), 6.6 g of toluene, 1.43 g of sodium tungstate dihydrate, and didecyldimethylammonium into a reaction vessel equipped with a thermometer, a stirrer, a reflux tube, and a dropping device. · 1.88 g of methyl sulfate, 0.69 g of phenylphosphonic acid, and 2.76 g of anhydrous sodium sulfate, after that, 32.7 g of 45% hydrogen peroxide solution was added dropwise over 4 hours while stirring at 25°C, and then carried out at 30°C 7 hours response. The pH of the reaction solution during the reaction was 4.7 to 6.5. After the reaction, gas chromatography was used to analyze, and the conversion rate of the compound represented by the result formula (1) was 97%, and the selectivity of the epoxy compound represented by the formula (2) to formula (4) was 92% (yield 90%) , the selectivity of by-products was 8% (yield 8%).
[0147]
Embodiment 3
[0149] Put 25.8 g of diene compounds represented by formula (1), 6.6 g of toluene, 1.43 g of sodium tungstate dihydrate, and didecyldimethylammonium into a reaction vessel equipped with a thermometer, a stirrer, a reflux pipe, and a dropping device. 1.93 g of methylsulfate and 0.72 g of phenylphosphonic acid were then added dropwise to 32.8 g of 45% aqueous hydrogen peroxide over 4 hours while stirring at 25° C., and then reacted at 30° C. for 7 hours. The pH of the reaction solution during the reaction was 4.1 to 5.3. After the reaction, gas chromatography was used to analyze, and the conversion rate of the compound represented by the result formula (1) was 99%, and the selectivity of the epoxy compound represented by the formula (2) to formula (4) was 84% (yield 83%) , the selectivity of by-products was 16% (yield 16%).
[0150]
PUM
| Property | Measurement | Unit |
|---|---|---|
| conversion efficiency | aaaaa | aaaaa |
| conversion efficiency | aaaaa | aaaaa |
| conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com