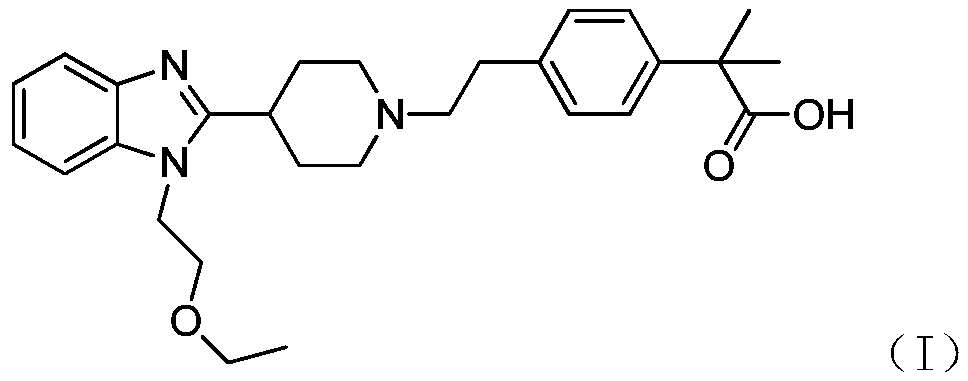
Preparation method of bilastine intermediate
A compound and reaction technology, applied in the field of preparation of bilastine intermediates, can solve the problems of increasing the amount of catalyst, severe reaction exotherm, affecting the reaction efficiency, etc., so as to improve experimental safety, reduce extraction times, and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
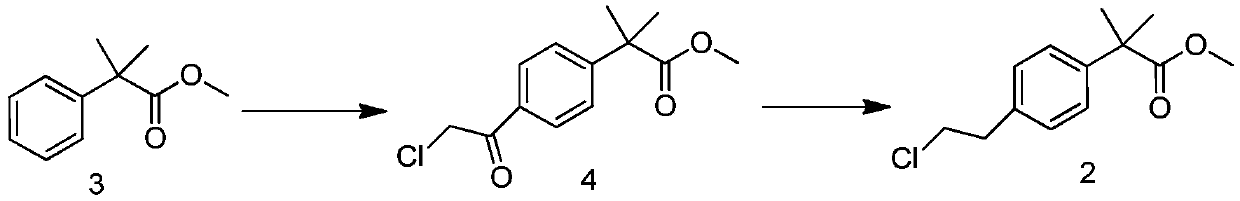
[0029] Embodiment 1 A kind of new preparation method of compound 2
[0030]
[0031] 1. Add 1000mL dichloromethane, 230.00g, 1.73mol anhydrous aluminum trichloride to the flask in turn;
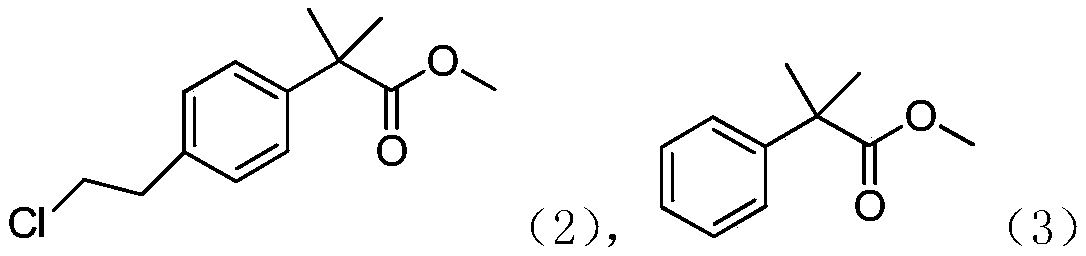
[0032] 2. At -5°C, slowly drop 100.00 g, 0.56 mol of compound 3 (dissolved in a small amount of dichloromethane) into the flask; then slowly drop 76.00 g, 0.67 mol of chloroacetyl chloride, and raise the temperature to 20 ℃ reaction, TCL monitors the reaction, and the reaction is completed in 3 hours;
[0033] 3. Cool the reaction solution to -5°C, slowly add 75.50 g, 0.56 mol 1,1,3,3-tetramethyldisiloxane dropwise, monitor the reaction with TCL, and complete the reaction in 1 hour;
[0034] 4. Cool down to -5°C, slowly drop the reaction solution into water to quench; separate the liquid, wash the organic phase once with saturated NaCl solution, dry, and distill off the solvent under reduced pressure to obtain a crude product; the crude product is distilled under reduced pressure again to...
Embodiment 2
[0037] Embodiment 2 A kind of new preparation method of compound 2
[0038] 1. Add 1000mL dichloromethane, 230.00g, 1.73mol anhydrous aluminum tribromide to the flask in turn;
[0039] 2. At -5°C, slowly drop 100.00g, 0.56mol of compound 3 (dissolved in a small amount of methylene chloride) into the flask; ℃ reaction, TCL monitors the reaction, and the reaction is completed in 3 hours;
[0040] 3. Cool the reaction liquid to -5°C, slowly add 75.50 g, 0.56 mol 1,1,3,3-tetramethyldisiloxane dropwise, monitor the reaction with TCL, and the reaction is completed in 1 hour;
[0041] 4. Cool down to -5°C, slowly drop the reaction solution into water to quench; separate the liquid, wash the organic phase once with saturated NaCl solution, dry, and distill off the solvent under reduced pressure to obtain a crude product; the crude product is distilled under reduced pressure again to obtain compound 2 The refined product 106.50g, GC detection purity 96.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com