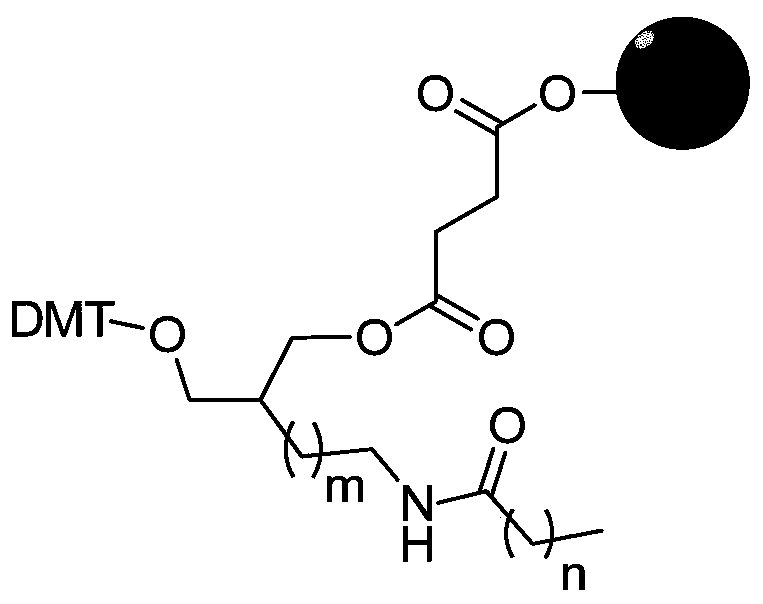
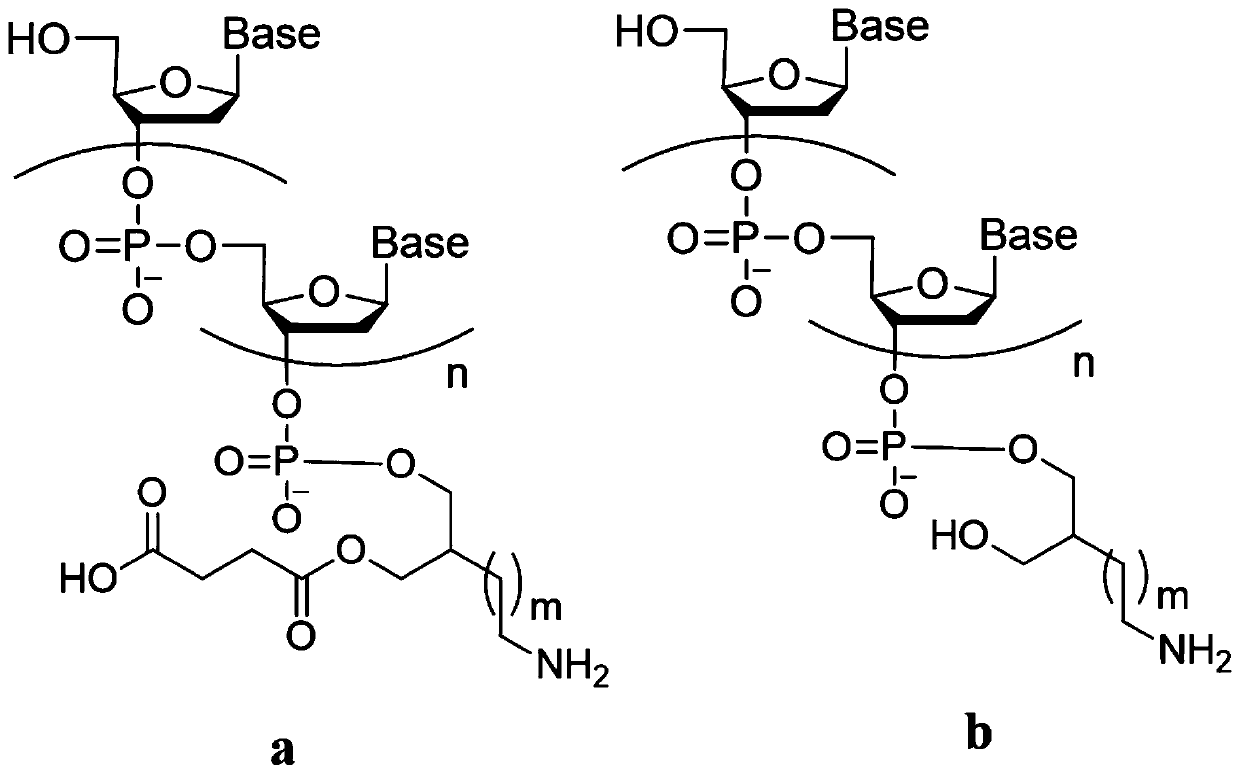
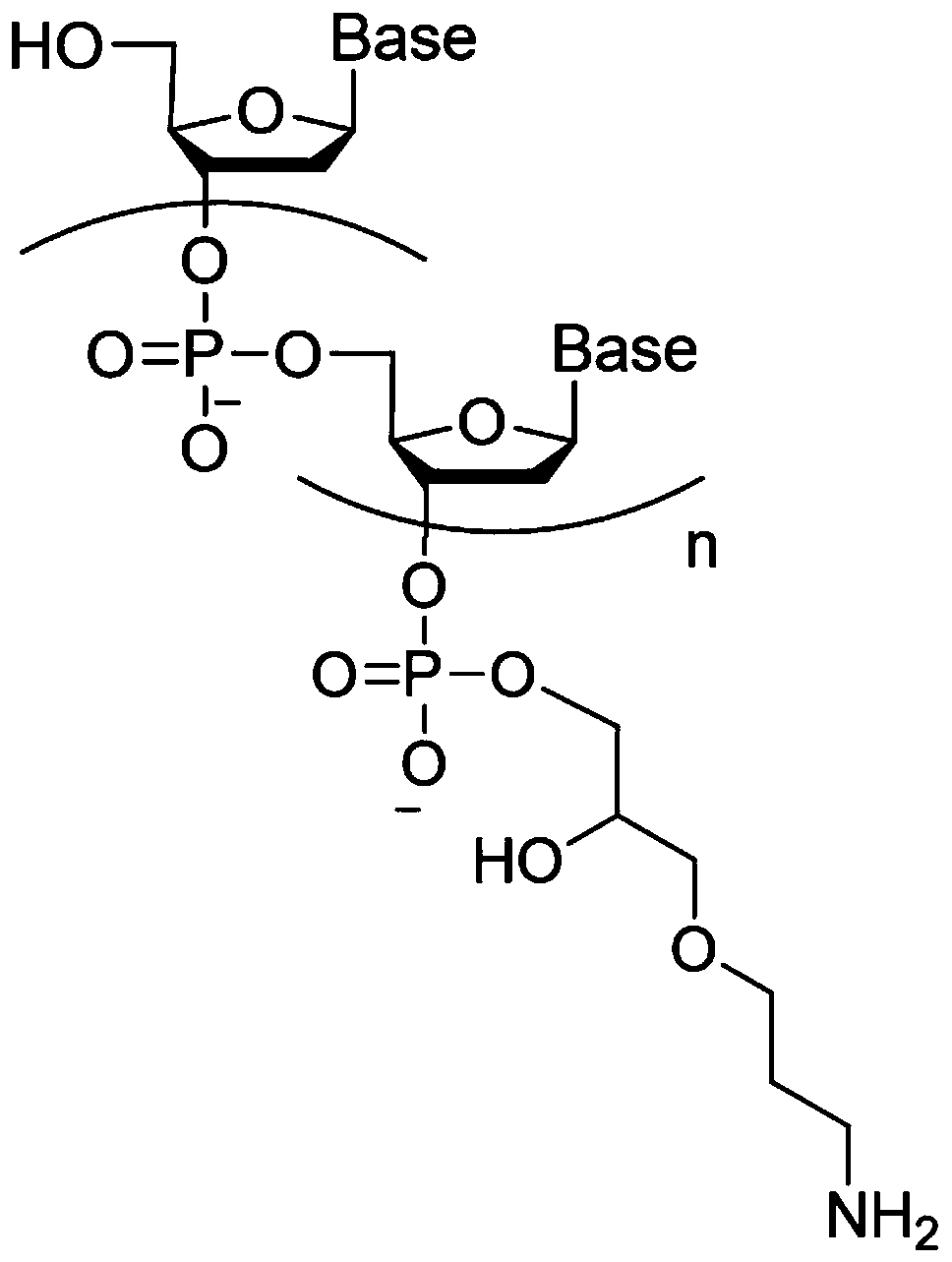
Connecting arm of immobilized nucleic acid aptamer as well as preparation method and application of the connecting arm
A nucleic acid aptamer and linker technology, which is used in the preparation of nucleic acid aptamer-drug conjugates. The linker field of nucleic acid aptamers can solve the problem of low selectivity and difficulty in obtaining linker raw materials, etc. problem, to achieve high selectivity, less side reactions, and easy to obtain effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0044] According to the present invention, the amino protecting group can be any group known in the art that can protect the amino to avoid chemical reaction. According to a preferred embodiment of the present invention, the amino protecting group is a halogen Substituted acetyl.
[0045] Wherein, the halogen may be chlorine or fluorine, preferably fluorine.
[0046] According to the present invention, the number of halogen substituents can be 1 or more, preferably 3.
[0047] According to a most preferred embodiment of the present invention, the amino protecting group is a trifluoroacetyl group.
[0048] According to the present invention, n can be any integer of 2, 3, 4, 5, 6, preferably, n is 2, 3 or 4.
[0049] In a second aspect, the present invention provides a method for preparing a tether of an immobilized nucleic acid aptamer, the method comprising:
[0050] (1) In the presence of the first catalyst, the nitrile of acrylonitrile or the substituted C3-C7 of the ω-ea...
Embodiment 1
[0149] This example is used to illustrate the synthesis of the tether of the nucleic acid aptamer immobilized provided by the present invention
[0150] (1) Synthesis of the first intermediate product
[0151]
[0152] Suspend 0.8g of sodium hydride (60% by weight, 20mmol) in 250mL of anhydrous tetrahydrofuran, stir in an ice-water bath, add 13.2g of compound 1 acetonide (100mmol) dropwise, after the addition is complete, gradually warm up to room temperature, and stir for 2 hours , add 13.2mL acrylonitrile (200mmol) dropwise under cooling in an ice-water bath, after the dropwise addition is complete, stir at room temperature for 2 hours, add 50mL of water, distill off THF under reduced pressure, extract with dichloromethane, collect the oil phase, concentrate, and distill under reduced pressure to obtain 16.8 g of oil (90.9% yield). 1 H NMR (400MHz, CDCl 3 ): δ1.35(s,3H),1.41(s,3H),2.61(t, J=6.3Hz,2H),3.51-3.58(m,2H),3.69-3.76(m,3H),4.05( dd, J=8.2Hz, J=6.4Hz, 1H), 4.23...
Embodiment 2
[0169] This example is used to illustrate the synthesis of the tether of the nucleic acid aptamer immobilized provided by the present invention
[0170] The synthesis of the linker arm of the immobilized nucleic acid aptamer was carried out according to the method in Example 1, except that in step (1), acrylonitrile was replaced with 4-bromobutyronitrile. The ammonolysis of a part of the product was taken and detected, which proved that the tether of the nucleic acid aptamer immobilized in the present invention was successfully obtained, and the yields of each step are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com