Process for refining low-molecular dermatan sulfate by enzyme-ultrafiltration method and application
A low-molecular-weight dermatan sulfate technology is applied to organic active ingredients, medical preparations containing active ingredients, blood diseases, etc. It can solve the problems of slow hydrolysis reaction, low bioavailability, excessive hydrolysis, etc. High product purity, high process repeatability, and low ultrafiltration loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
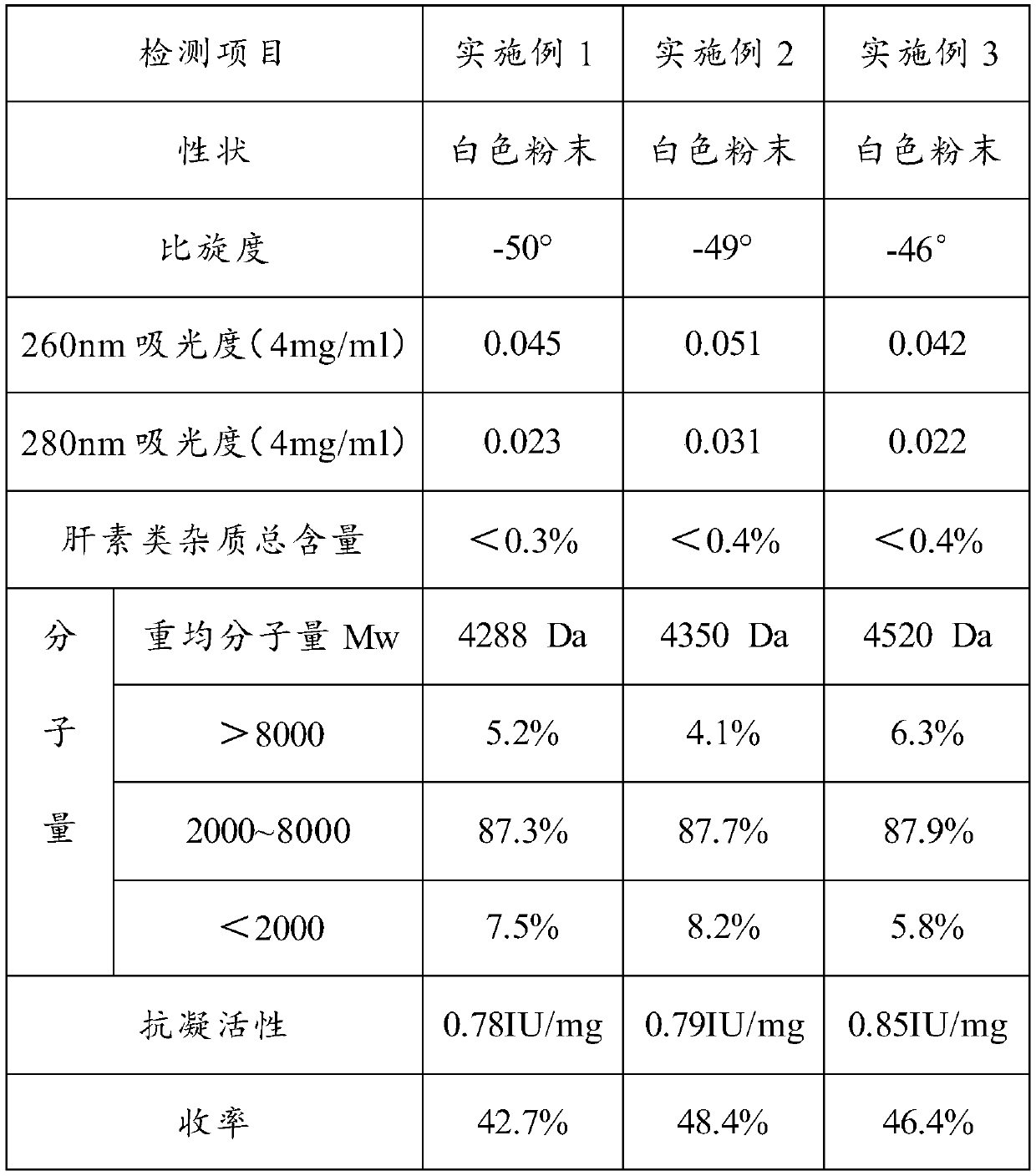
Embodiment 1
[0155] Weigh 1Kg of heparin sodium by-product and add 10L of water to dissolve it into a feed liquid with a concentration of about 10%, raise the temperature of the feed liquid to 60°C, and slowly add about 10L of resin to the resin column for resin adsorption; after the adsorption is completed, use 25L concentration of 3.5% sodium chloride solution, adjust the temperature to 60°C for elution, discard the eluent; then use 30L concentration of 8% sodium chloride solution (adjust temperature to 60°C) for elution, Collect this part of the eluate to the 1# tank; after the elution is completed, accurately measure the volume of the collected eluate to be 32L, add 12.8L of ethanol, stir evenly, let it stand for 3 hours, and transfer the supernatant suspension to the 2# tank (the sediment at the bottom of the 1# tank is heparinoid), add 64L of ethanol to the 2# tank again, stir evenly, let it stand for 6 hours, discard the supernatant, collect the sediment at the bottom of the 2# tank,...
Embodiment 2
[0164] Weigh 50Kg of the heparin sodium by-product and add 500L water to dissolve it, and dissolve it into a feed liquid with a concentration of about 10%, raise the temperature of the feed liquid to 60°C, slowly add about 500L resin to the resin column, and perform resin adsorption; after the adsorption is completed, use 1250L concentration of 3.5% sodium chloride solution, adjust the temperature to 60°C for elution, discard the eluent; then use 1500L concentration of 8% sodium chloride solution (adjust temperature to 60°C) for elution, Collect this part of the eluate to the 5# tank; after the elution is completed, accurately measure the volume of the collected eluate to be 1600L, add 640L of ethanol, stir evenly, let stand for 3h, and transfer the supernatant suspension to the 6# tank (The sediment at the bottom of the 5# tank is heparinoid), add 3000L ethanol to the 6# tank again, stir evenly, let it stand for 8 hours, discard the supernatant, collect the sediment at the bot...
Embodiment 3
[0170] Weigh 100Kg of the heparin sodium by-product and add 1000L water to dissolve it, and dissolve it into a feed liquid with a concentration of about 10%, raise the temperature of the feed liquid to 60°C, and slowly add about 1000L of resin to the resin column for resin adsorption; after the adsorption is completed, use 2500L concentration of 3.5% sodium chloride solution, adjust the temperature to 60°C for elution, discard the eluent; then use 3000L concentration of 8% sodium chloride solution (adjust temperature to 60°C) for elution, Collect this part of the eluate to the 5# tank; after the elution is completed, accurately measure the volume of the collected eluate to be 3200L, add 1280L of ethanol, stir evenly, let stand for 3h, and transfer the supernatant suspension to the 6# tank (The sediment at the bottom of the 5# tank is heparinoid), add 6000L ethanol to the 6# tank again, stir evenly, let it stand for 8 hours, discard the supernatant, collect the sediment at the b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com