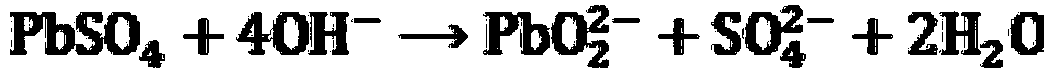Recovery method of waste lead-acid battery
A recycling method, waste lead-acid technology, applied in battery recycling, lead-acid batteries, waste collector recycling, etc., can solve the problems of waste lead-acid battery recycling method is not clean enough, to achieve more atomic economy, simple operation steps, The effect of broad market prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
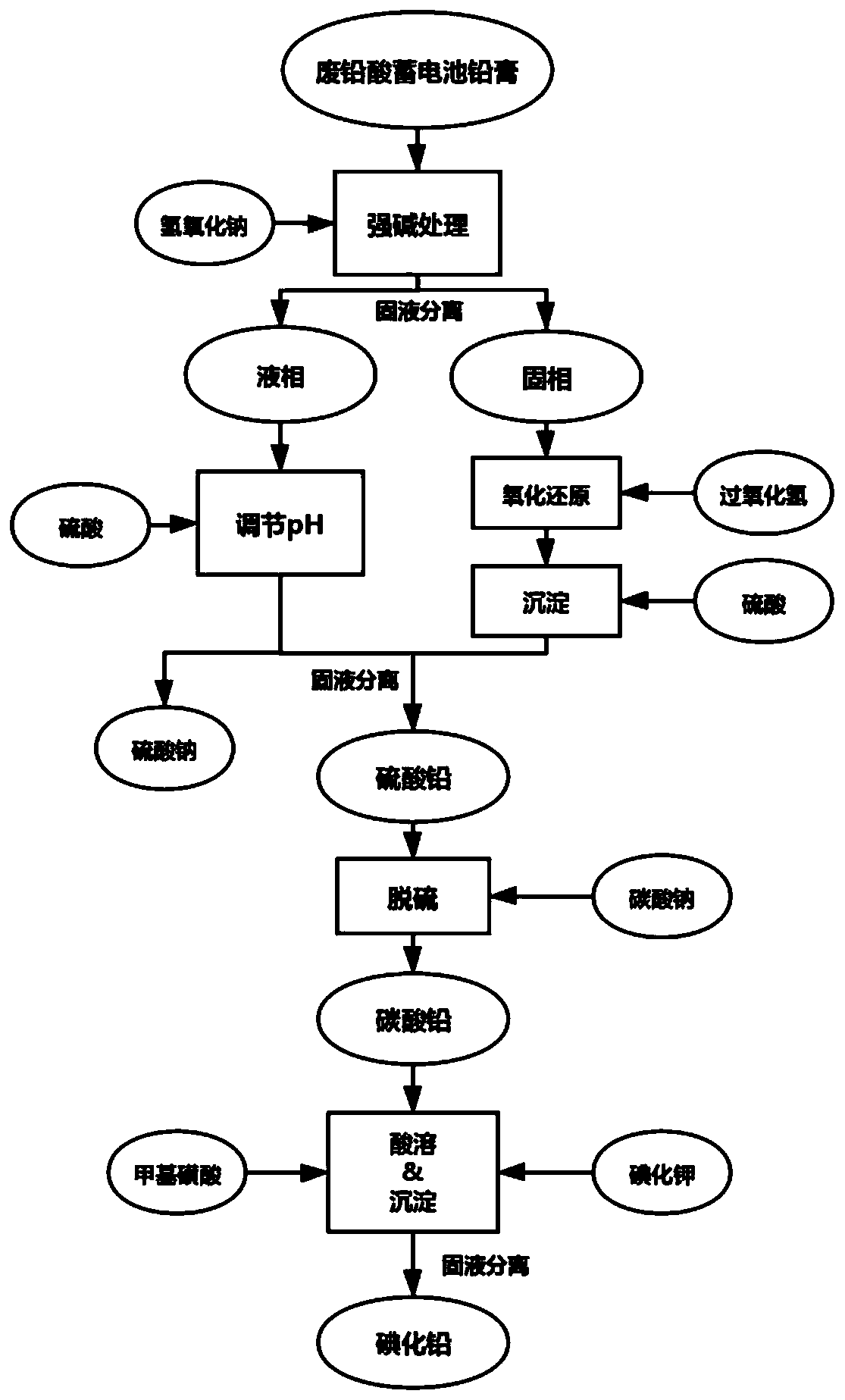
[0097] Take 100g of the lead paste obtained from the waste lead-acid battery, use 1L of NaOH solution with a concentration of 2mol / L to extract it, fully stir for 1h and then filter. The filter residue obtained in the above-mentioned filtration process first takes 0.5mol / L H with a pH of 2 2 O 2 500mL of the solution was reacted (using methanesulfonic acid to adjust the pH value of the solution), and then 150mL of a sulfuric acid aqueous solution with a concentration of 2mol / L was added to the filtrate obtained after the reaction and stirred, and then filtered, washed and dried to obtain high-purity lead sulfate . The filtrate obtained by leaching the lead paste with the above NaOH solution and separating the solid-liquid is then added dropwise concentrated sulfuric acid and fully stirred to adjust the pH of the solution to about 3, filtered, washed and dried to obtain high-purity sulfuric acid. lead. After mixing the lead sulfate obtained in the above two different processes,...
Embodiment 2
[0100] Take 250g of the lead paste obtained from the waste lead-acid battery, use 3L of NaOH solution with a concentration of 2mol / L to extract it, fully stir for 1h and 20min, and then filter. The filter residue obtained in the above filtration process first takes 0.5mol / L H with a pH of 1.5 2 O 2 The solution is 1.5L for reaction (using methanesulfonic acid to adjust the pH value of the solution), and then 500mL of a sulfuric acid aqueous solution with a concentration of 2mol / L is added to the filtrate obtained after the reaction and stirred, and then filtered, washed and dried to obtain high-purity sulfuric acid lead. The filtrate obtained by leaching the lead paste with the above NaOH solution and separating the solid-liquid is then added dropwise concentrated sulfuric acid and fully stirred to adjust the pH of the solution to about 3, filtered, washed and dried to obtain high-purity sulfuric acid. lead. After mixing the lead sulfate obtained in the above two different pro...
Embodiment 3
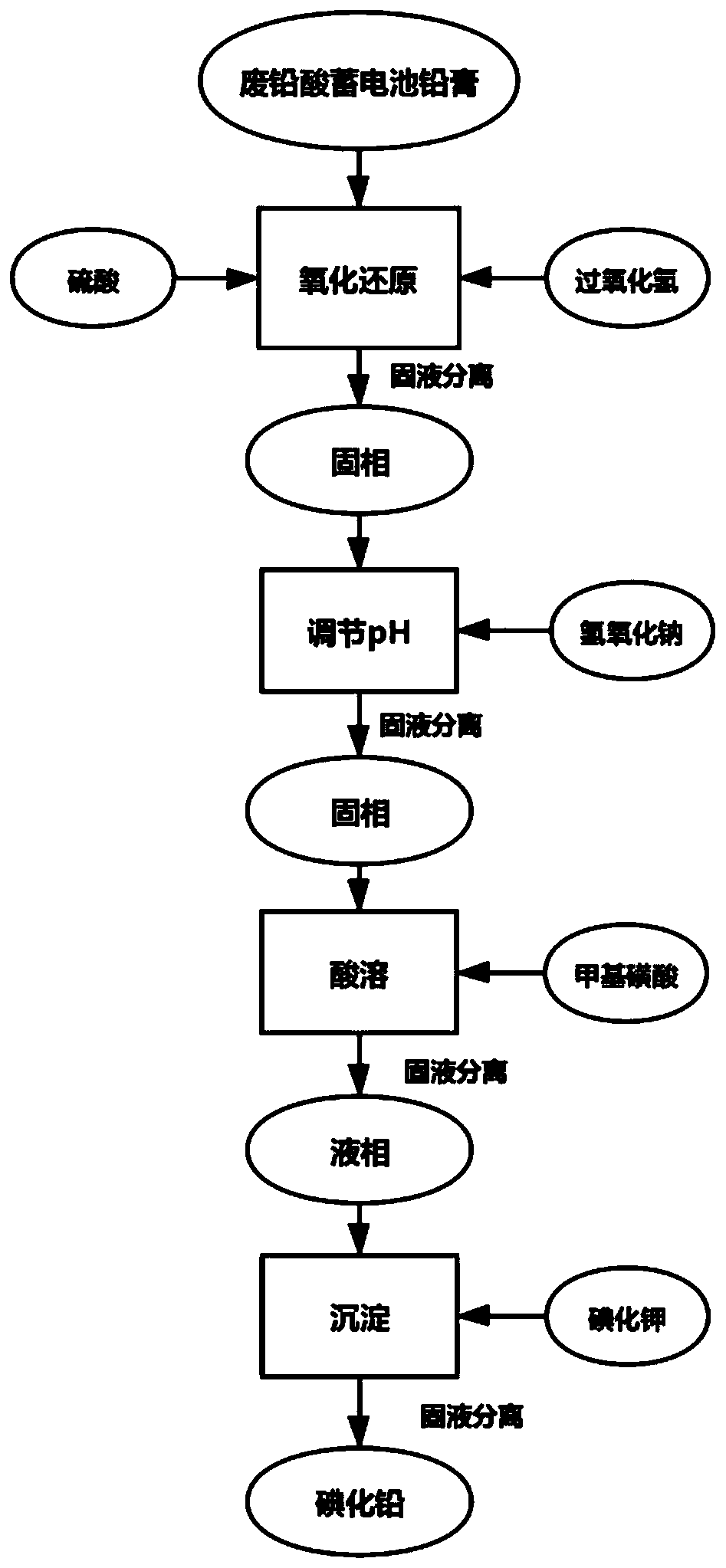
[0103] Take 100g of lead paste obtained from waste lead-acid batteries, add 250mL of sulfuric acid with a concentration of 2mol / L and 100mL of aqueous hydrogen peroxide solution with a concentration of 1mol / L into it to make 1000ml aqueous solution, and extract under stirring 1h. After solid-liquid separation, add 2L of sodium hydroxide solution with a concentration of 0.5mol / L to the filter residue, adjust the pH to about 8 with sulfuric acid, leaching for 1h under stirring, and after filtering and separating, add 400mL of sodium hydroxide to the white precipitate 2.1mol of methanesulfonic acid was continuously stirred under the condition of operating temperature of 60℃. After the white precipitate was completely dissolved, the concentration was adjusted to obtain 1mol / L of lead methanesulfonate, and then 400mL of 2.2mol / L was added. The potassium iodide solution was stirred and then separated by filtration. The separated solid phase was washed 5 times with deionized water, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com