Medicine composition for preventing and treating diabetes mellitus and application of medicine composition
A composition and technology for diabetes, applied in the directions of drug combination, urinary system diseases, metabolic diseases, etc., can solve the problems such as the prevention and treatment of diabetes that have not been reported with rebamipide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: The effect of the composition of rebamipide and asiaticoside and its analogs on blood sugar in NOD mice
[0049] 1. Experimental animals and experimental materials
[0050] SPF grade NOD mice, female, 4 weeks old, were purchased from Beijing Huafukang Biotechnology Co., Ltd.
[0051] Rebapait: Aladdin, China
[0052] Asiaticoside: MedChemExpress Reagent Company, USA
[0053] Madecassoside: MedChemExpress Reagents, USA
[0054] Asiatic acid: Aladdin, China
[0055] Madecassic acid: Aladdin, China
[0056] Sodium carboxymethylcellulose: Aladdin, China
[0057] Solvent used in the control group: 0.5% CMC-Na
[0058] 2. Experimental process
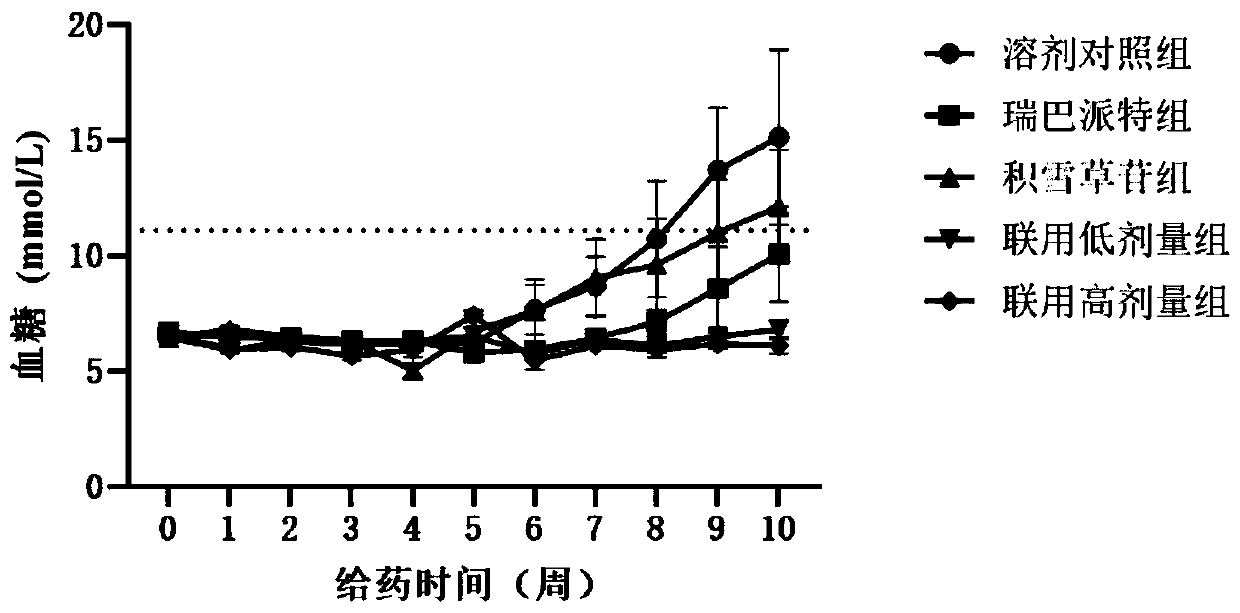
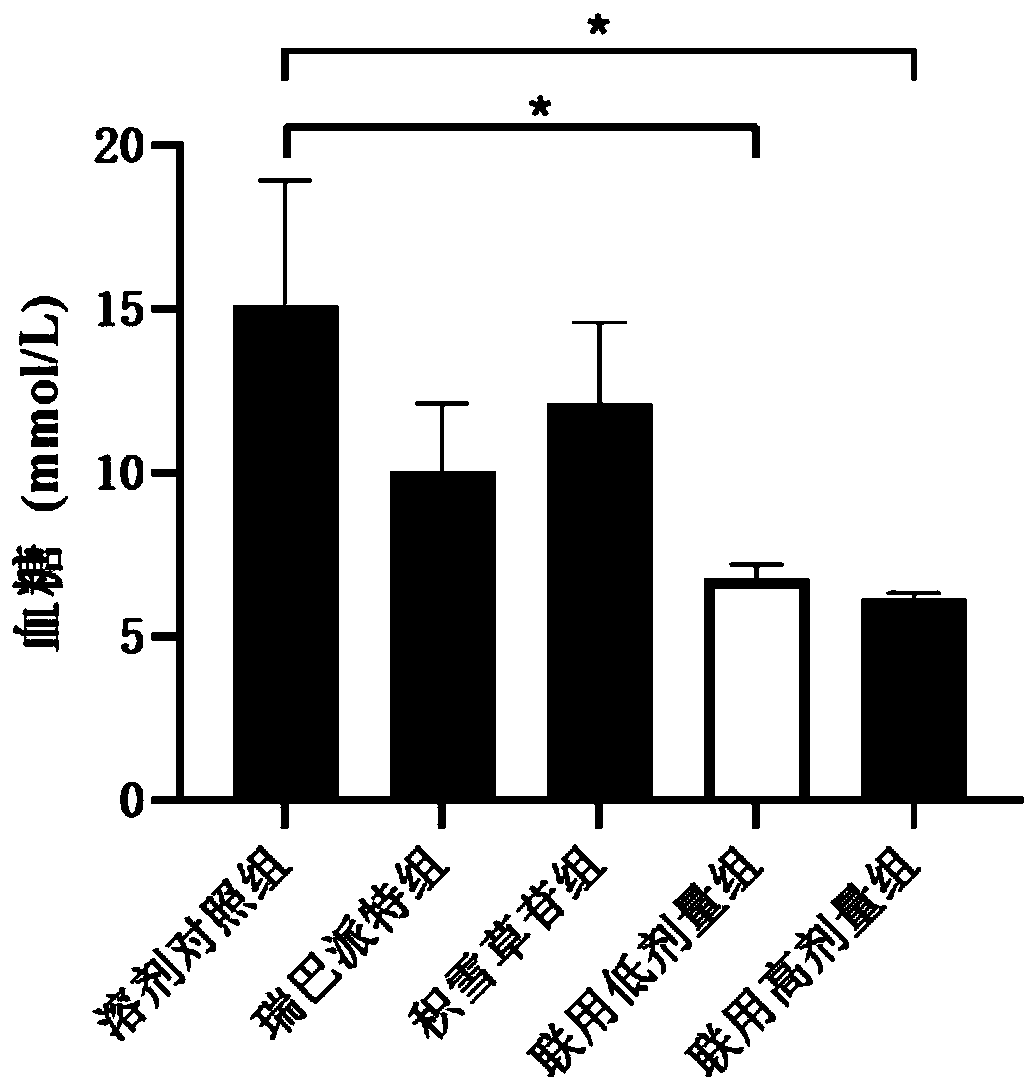
[0059] After adaptive feeding for 1 week, the random blood glucose value of the tail vein of the mice was measured with a blood glucose meter at 9:00 a.m. every day for 3 consecutive measurements. According to the random blood sugar and body weight, the mice were randomly divided into groups and dosed, administered o...
Embodiment 2
[0065] Embodiment 2: Drug effect evaluation of the pharmaceutical composition of the present invention on type 1 diabetes NOD mice
[0066] 1. Experimental animals
[0067] SPF grade NOD mice, female, 4 weeks old, were purchased from Beijing Huafukang Biotechnology Co., Ltd. All animals were raised in an IVC environment, provided with sufficient feed and drinking water, maintained a circadian rhythm of 12 hours of light and 12 hours of darkness, with a temperature of 25±2°C and a humidity of 50%-70%.
[0068] All animal experiments were performed in accordance with the relevant experimental regulations of China Pharmaceutical University, and the experimental process followed animal ethics.
[0069] 2. Main reagents
[0070] Rebapait: Aladdin, China
[0071] Asiaticoside: MedChemExpress Reagent Company, USA
[0072] Sodium carboxymethylcellulose: Aladdin, China
[0073] 3. Experimental process
[0074] After the NOD mice were adaptively fed for 1 week, the random blood gl...
Embodiment 3
[0125] Embodiment 3: The drug effect evaluation of pharmaceutical composition of the present invention to type 2 diabetes db / db mice
[0126] 1. Experimental animals
[0127] SPF-grade db / db mice, male, 4 weeks old, were purchased from the Institute of Model Animals, Nanjing University.
[0128] 2. Main reagents
[0129] Metformin: Aladdin, China
[0130] Rebapait: Aladdin, China
[0131] Asiaticoside: MedChemExpress Reagent Company, USA
[0132] Sodium carboxymethylcellulose: Aladdin, China
[0133] 3. Experimental process
[0134] (1) Grouping and administration:
[0135] The db / db mice were adaptively fed for 1 week, and then randomly grouped according to their fasting blood glucose and body weight:
[0136] A. Solvent control group: 0.5% CMC-Na, 10 rats;
[0137] B. Metformin group: 150mg / kg, 10 rats;
[0138] C. Rebamipide group: 150mg / kg, 10 rats;
[0139] D. Asiaticoside group: 60mg / kg, 10 rats;
[0140] E. Combined low-dose group: rebamipide 75mg / kg+ asiatic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com