Tetracyano-anthraquinone dimethane micromolecular receptor material as well as preparation method and application thereof
A technology of tetracyanoanthraquinone dimethane and small molecules, which is applied in the direction of luminescent materials, carboxylic acid nitrile preparation, chemical instruments and methods, etc. It can solve the problems of poor solubility of compounds, application limitations, and low charge carrier mobility. , to achieve the effects of good matching, good solubility, and improved electron transport performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
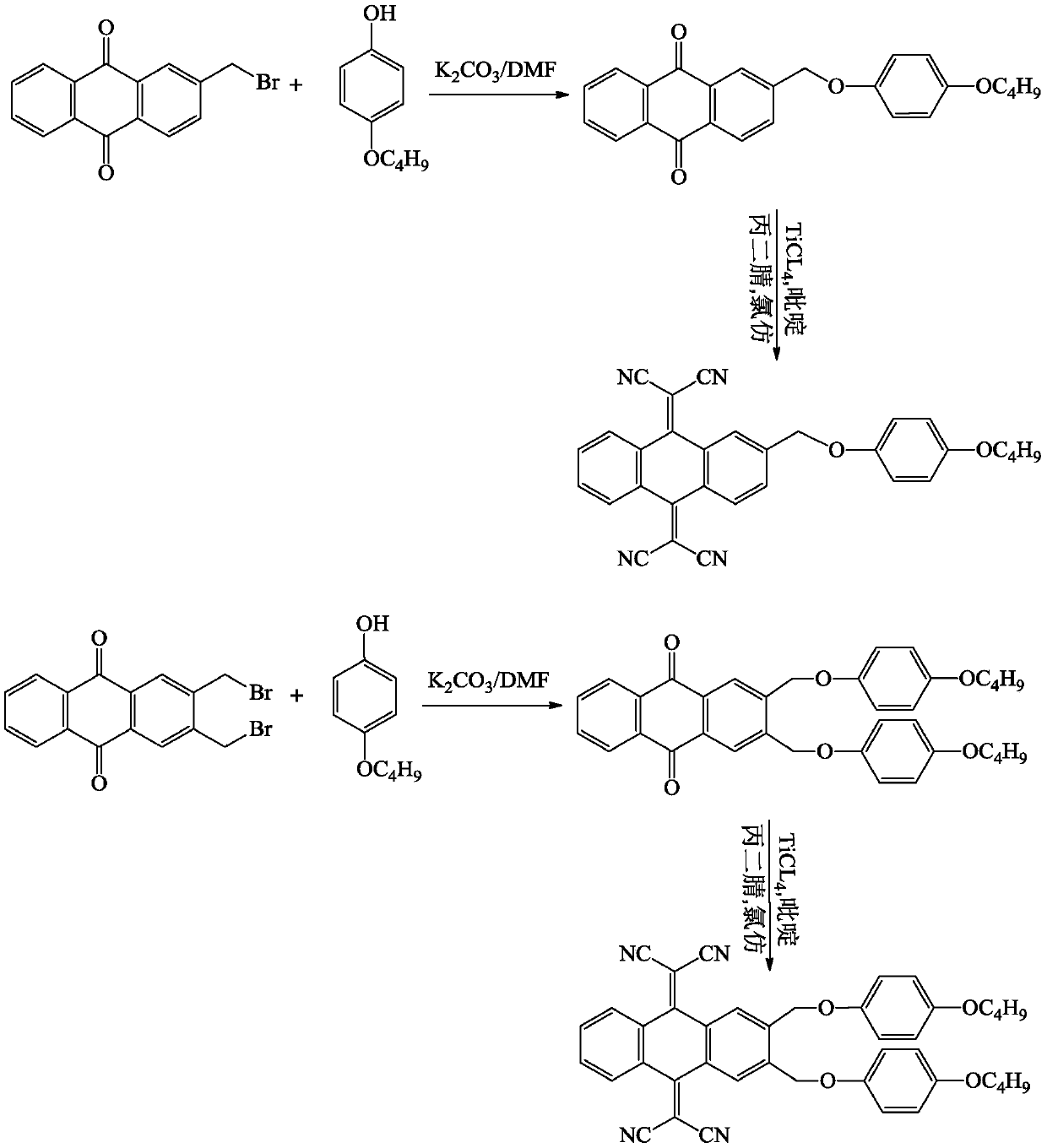
[0059] Example 1 Compound I-1: Synthesis of 11,11,12,12-tetracyano-2-(4`-butoxyphenoxymethyl)-9,10-anthraquinone dimethane (prepared according to Scheme 1 get)
[0060] 1.1 Synthesis of 4-butoxyphenol
[0061]
[0062] Add 26.4 g (0.24 mol) of hydroquinone, 27.4 g (0.2 mol) of n-bromobutane, 8.8 g (0.22 mol) of sodium hydroxide and 125 mL of N,N-dimethylformamide In a 500mL round-bottomed flask, heat to 60°C for 12 hours; naturally cool to room temperature, pour into 500mL of ice water, extract with ethyl acetate (3×100mL), and wash the organic phase with water (3×100mL). Magnesium sulfate water was dried overnight, and the solid obtained after the solvent was removed was recrystallized with petroleum ether, suction filtered, and vacuum-dried at 50°C for 10 hours to obtain 11.3 grams of product, the yield was 34%, and the melting point was 62-64°C; IR(KBr Tablet, cm -1 ):3404.36,3354.21,2956.87,2931.8,2916.37,2872.01,2374.37,2345.44,1606.7,1541.12,1514.12,1477.47,1456.26...
Embodiment 2
[0078] Example 2 Compound I-1: Synthesis of 11,11,12,12-tetracyano-2-(4`-butoxyphenoxymethyl)-9,10-anthraquinone dimethane (prepared according to Scheme 2 get)
[0079] 2.1 Synthesis of 4-butoxyphenol
[0080]
[0081] Add 26.4 g (0.24 mol) of hydroquinone, 27.4 g (0.2 mol) of n-bromobutane, 8.8 g (0.22 mol) of sodium hydroxide and 125 mL of N,N-dimethylformamide In a 500mL round-bottomed flask, heat to 60°C for 12 hours; naturally cool to room temperature, pour into 500mL of ice water, extract with ethyl acetate (3×100mL), and wash the organic phase with water (3×100mL). Magnesium sulfate water was dried overnight, and the solid obtained after the solvent was removed was recrystallized with petroleum ether, suction filtered, and vacuum-dried at 50°C for 10 hours to obtain 11.3 grams of product, the yield was 34%, and the melting point was 62-64°C; IR(KBr Tablet, cm -1 ):3404.36,3354.21,2956.87,2931.8,2916.37,2872.01,2374.37,2345.44,1606.7,1541.12,1514.12,1477.47,1456.26...
Embodiment 3
[0098] With reference to the synthetic method of embodiment 1 or embodiment 2, the specific compound structure is synthesized, as shown in the following table:
[0099]
[0100]
[0101] Table 1
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com