CuOx nano-cluster and application thereof as ozone catalyst
A nano-cluster and reaction technology, which is applied in the direction of metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, copper oxide/copper hydroxide, etc. Problems such as high surface energy, to achieve a simple and controllable preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] a CuO x Nanoclusters, the preparation process is as follows:
[0036] (1) Add 0.05g TiO 2 The nanotubes were added to 20 mL of ethanol, and 200 μL of 3-aminopropyltrimethoxysilane was added while stirring, stirred and reacted for 12 hours, filtered and washed to obtain a surface-aminated carrier;
[0037] (2) Add the surface-aminated carrier to 50 mL of copper ion solution with a copper ion concentration of 10 mg / L, stir for 20 seconds, filter, wash, and dry;
[0038] (3) Place the dried material in a muffle furnace, and after calcination at a calcination temperature of 350°C for 3 hours, cool to room temperature to obtain a CuO x nanoclusters.
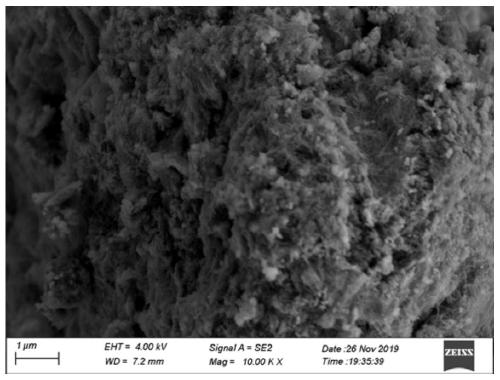
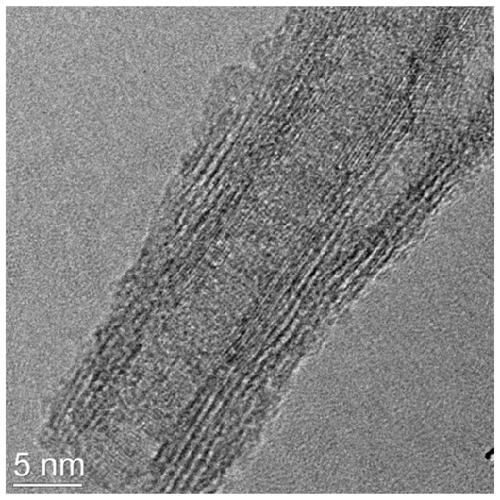
[0039] Scanning electron microscopy of the final product as prepared figure 1 shown, from figure 1 Only the nanotube structure of the support can be seen in ; the high-resolution transmission electron microscope of the prepared final product is shown in figure 2 As shown, when the scale bar is 5nm, the diffraction fringe...
Embodiment 2
[0042] a CuO x Nanoclusters, the preparation process is as follows:
[0043] (1) 0.05g CeO 2 The nanotubes were added to 5 mL of ethanol, and 500 μL of 3-aminopropyltrimethoxysilane was added while stirring, stirred and reacted for 12 hours, filtered and washed to obtain a surface-aminated carrier;
[0044] (2) Add the surface-aminated carrier to 33 mL of copper ion solution with a copper ion concentration of 20 mg / L, stir for 10 seconds, filter, wash, and dry;
[0045] (3) The dried material was placed in a muffle furnace, calcined at a calcination temperature of 350° C. for 3 h, and then cooled to room temperature to obtain a CuOx nanocluster.
[0046] Catalyst performance evaluation: the antibiotic florfenicol was selected as the simulated pollutant, but not limited to florfenicol, and 100mL, 15mg / L florfenicol wastewater, 0.3g / L CeO 2 Nanotube-supported CuO x Cluster ozone catalyst, test the removal rate of florfenicol to evaluate the catalyst's ozone catalytic oxidati...
Embodiment 3
[0050] a CuO x Nanoclusters, the preparation process is as follows:
[0051] (1) Add 0.05g TiO 2 The nanotubes were added to 20 mL of ethanol, and 200 μL of 3-aminopropyltrimethoxysilane was added while stirring, stirred and reacted for 12 hours, filtered and washed to obtain a surface-aminated carrier;
[0052] (2) Add the surface-aminated carrier to 1000 mL of copper ion solution with a copper ion concentration of 1 mg / L, stir for 30 seconds, filter, wash, and dry;
[0053] (3) Place the dried material in a muffle furnace, and after calcination at a calcination temperature of 350°C for 3 hours, cool to room temperature to obtain a CuO x nanoclusters.
[0054] Catalyst performance evaluation: the antibiotic florfenicol was selected as the simulated pollutant, but not limited to florfenicol, and 100mL, 15mg / L florfenicol wastewater, 0.3g / L TiO 2 Nanotube-supported CuO x Cluster ozone catalyst, test the removal rate of florfenicol to evaluate the catalyst's ozone catalytic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com