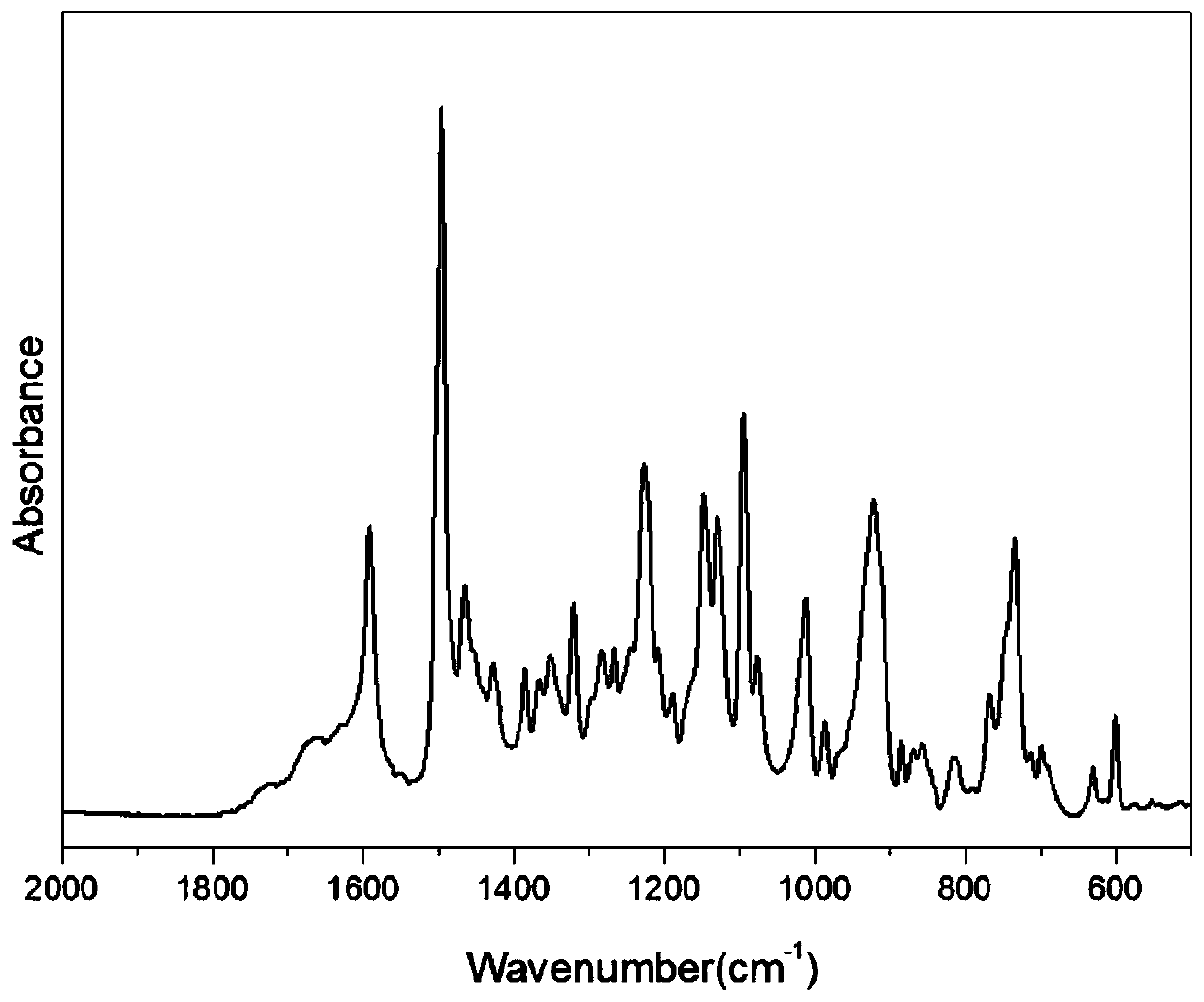
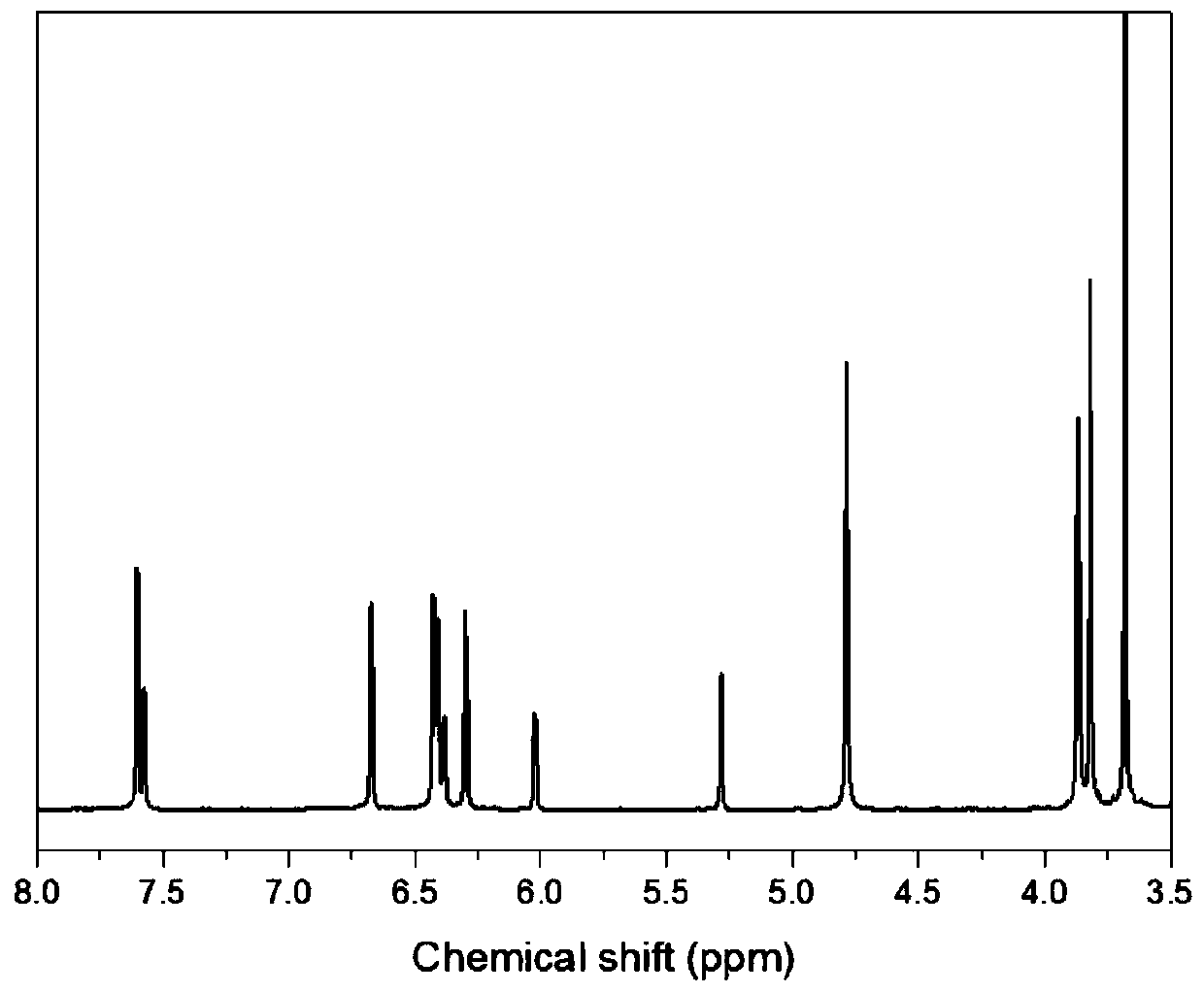
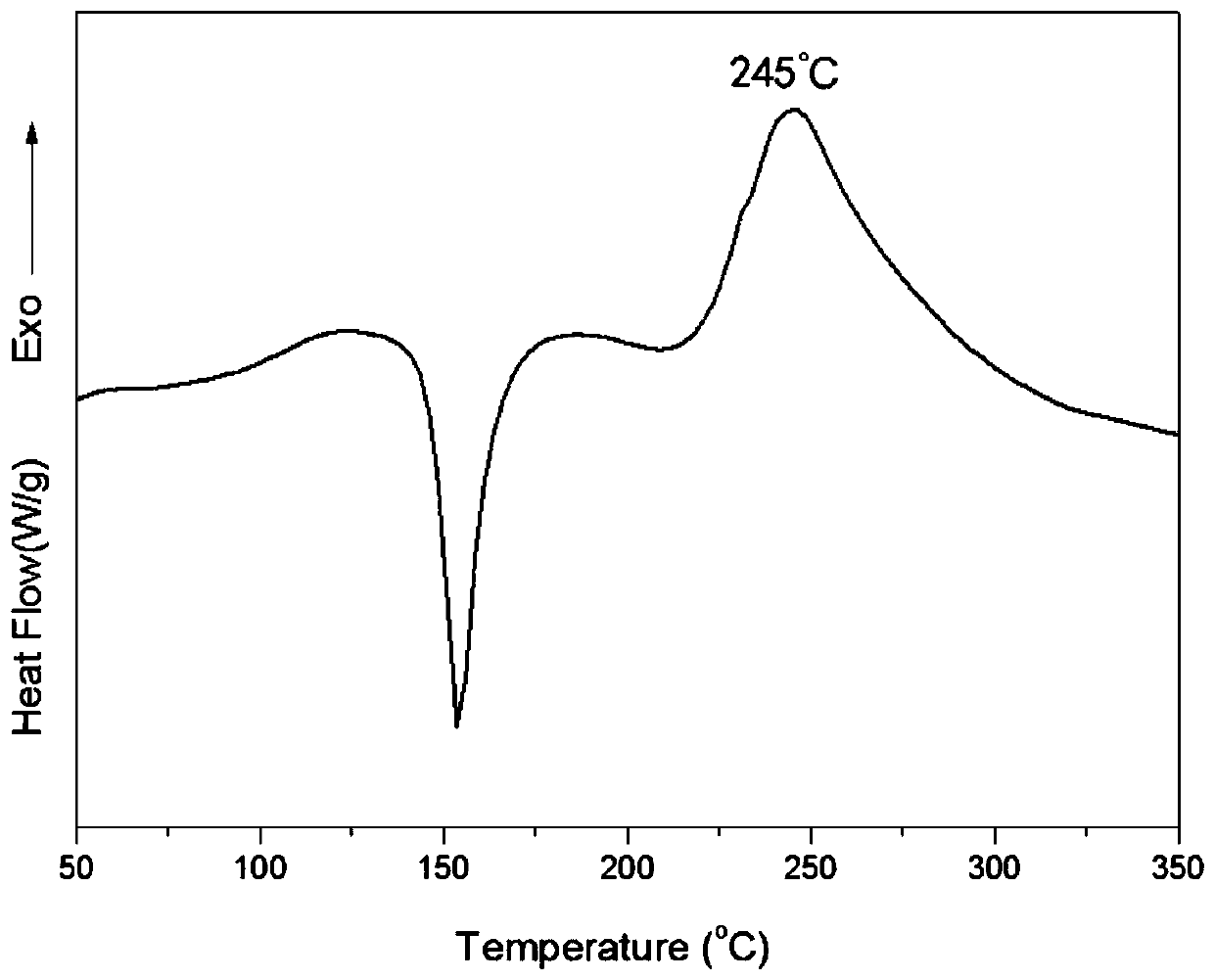
Bio-based dibenzoxazine monomer and preparation method thereof
A benzoxazine and bio-based technology, applied in the field of thermosetting resin and its preparation, can solve the problems of limited application prospects, poor mechanical properties and thermal stability, etc., and achieve simple synthesis process, low equipment requirements, and excellent heat resistance and the effect of flame retardancy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) 4.966g (0.04mol) of guaiacol, 1.922g (0.02mol) of furfural, and a 20% sodium hydroxide solution (wherein the quality of sodium hydroxide is 0.496g) in the flask are added into the flask, React at 60°C for 8 hours, then raise the temperature to 120°C and continue to react for 12 hours. After the reaction, it was washed with alkali and purified by acidification to obtain 4.89 g of intermediate bio-based bisphenol with a yield of 75%. The chemical reaction equation is as follows:
[0034]
[0035] (2) Add 1.63g (0.005mol) of bio-based bisphenol obtained in step (1), 0.97g (0.01mol) of 2-furylmethylamine, 0.868g (0.0289mol) of paraformaldehyde into the flask, and add 50ml of toluene solution , Connected to a condenser, stirred and reacted at 110°C for 4h. The filtrate after the reaction was rotary evaporated to remove the solvent to obtain 2.27 g of benzoxazine monomer with a yield of 80%. The chemical reaction equation is as follows:
[0036]
[0037] In the ...
Embodiment 2
[0041] The reactant 2-furan methylamine of step (2) in embodiment 1 is replaced with 3-methylaniline, and the amount of reactant is changed accordingly, and other operating steps are with the steps in embodiment 1.
[0042] In the second step reaction, the amount of the reactant was changed to: 1.63g (0.005mol) of bio-based bisphenol obtained from the previous step reaction, 1.07g (0.01mol) of 3-methylaniline and 0.868 (0.0289mol) of paraformaldehyde ) to react, finally obtain product 2.29g, yield 78%.
[0043] Wherein the specific chemical structure of the benzoxazine monomer obtained is:
[0044]
[0045] The exothermic peak temperature of the curing benzoxazine obtained in this example is 251°C. After further curing and cross-linking, the temperature of the polybenzoxazine resin is 366°C when the thermal weight loss is 10%. When the inert gas atmosphere is 800°C, the carbon residue The rate is 61%, and the heat release energy of the flame retardant test result is 89Jg ...
Embodiment 3
[0047] The reactant 2-furyl methylamine in the second step in the embodiment 1 is replaced with 3-fluoroaniline, and the amount of the reactant is changed accordingly, and other operation steps are with the steps in the embodiment 1.
[0048]In the second step reaction, the amount of the reactant was changed to: 1.63g (0.005mol) of bio-based bisphenol obtained from the previous step reaction, 1.11g (0.01mol) of 3-methylaniline and 0.868 (0.0289mol) of paraformaldehyde ) to react to obtain product 2.15g, yield 72%.
[0049] Wherein the specific chemical structure of the benzoxazine monomer obtained is:
[0050]
[0051] The exothermic peak temperature of the curing benzoxazine obtained in this example is 258°C. After further curing and crosslinking, the temperature of the polybenzoxazine resin is 371°C when the thermal weight loss is 10%. When the inert gas atmosphere is 800°C, the carbon residue The rate is 63%, and the heat release energy of the flame retardant test resul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com