Alcohol dehydrogenase mutant and application thereof in synthesis of chiral diaryl alcohol compounds
A technology of alcohol dehydrogenase and catalytic center, applied in application, antibody mimics/scaffolds, enzymes, etc., can solve the problems of unknown protein sequence, high stereoselectivity, and no amino acid sequence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
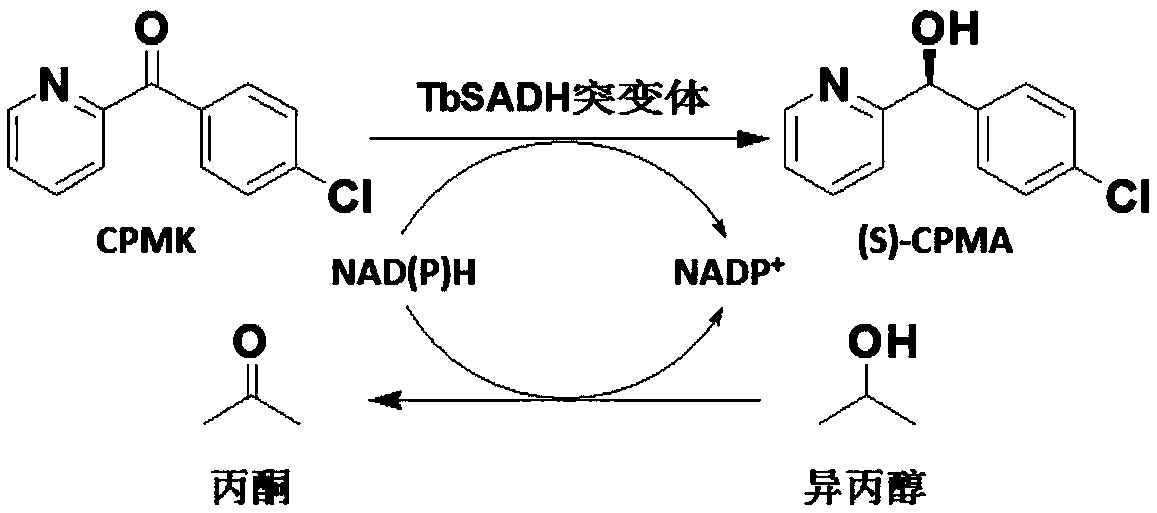
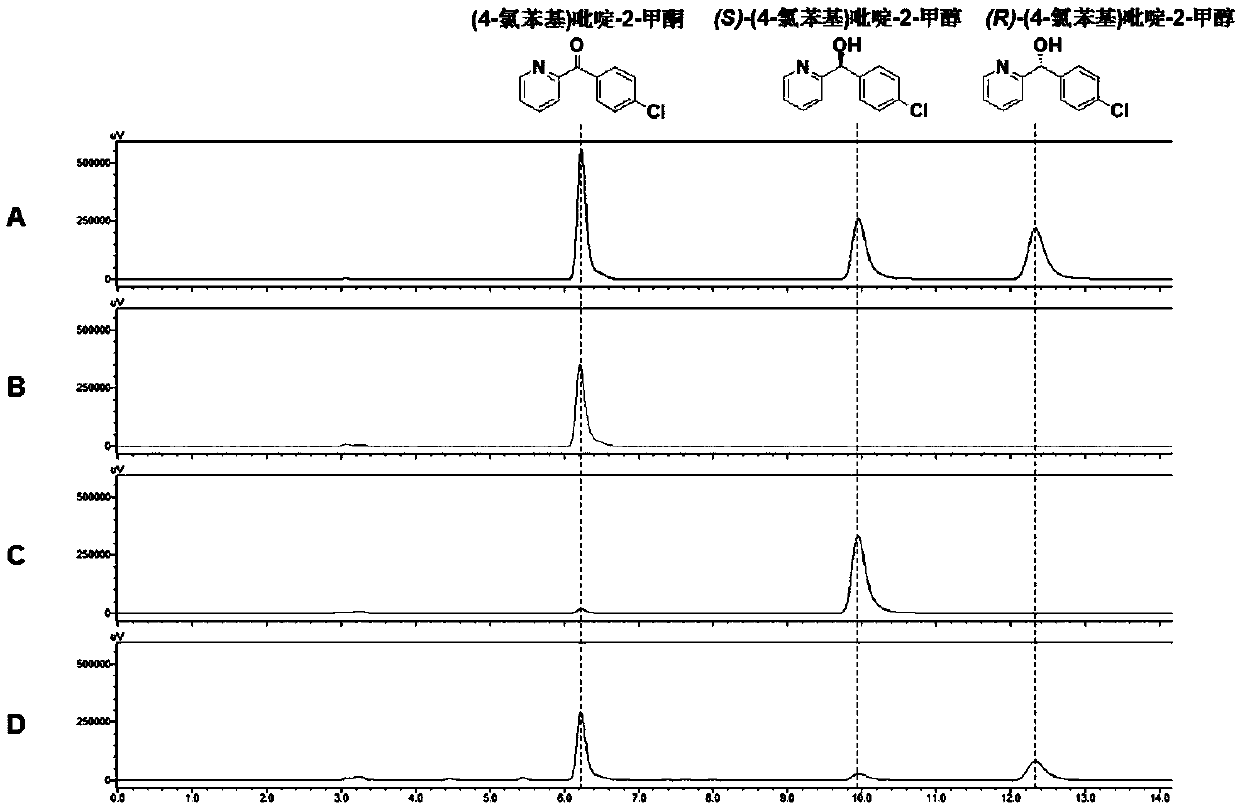
[0149] Embodiment 1, alcohol dehydrogenase TbSADH and its mutant sequence
[0150] The amino acid sequence of the alcohol dehydrogenase TbSADH in this example is shown in SEQ ID No.2. In this embodiment, the alcohol dehydrogenase TbSADH mutant is specifically any of the following (1)-(39):
[0151] (1) Alcohol dehydrogenase TbSADH mutant P84A: After mutating the 84th amino acid of the amino acid sequence of alcohol dehydrogenase TbSADH from proline (P) to alanine (A), and keeping other amino acid sequences unchanged The protein which becomes.
[0152] (2) Alcohol dehydrogenase TbSADH mutant P84V: After mutating the 84th amino acid of the amino acid sequence of alcohol dehydrogenase TbSADH from proline (P) to valine (V), and keeping other amino acid sequences unchanged The protein which becomes.
[0153] (3) Alcohol dehydrogenase TbSADH mutant P84S: obtained by mutating the 84th amino acid of the amino acid sequence of alcohol dehydrogenase TbSADH from proline (P) to serine ...
Embodiment 2
[0190] Embodiment 2, the preparation of the engineering bacterium of alcohol dehydrogenase TbSADH gene or its mutant
[0191] 1. Preparation of Alcohol Dehydrogenase TbSADH Gene Wild Type Genetic Engineering Strain
[0192] 1. Optimization of Alcohol Dehydrogenase TbSADH Gene Sequence
[0193] The alcohol dehydrogenase TbSADH gene derived from Thermoanaerobacter brockii was codon-optimized with Escherichia coli as the host cell, and the whole gene was synthesized after optimization. The optimized alcohol dehydrogenase TbSADH gene sequence is shown as SEQ ID No. 1.
[0194] 2. Construction of recombinant vector pRSFDuet-1-TbSADH
[0195] Use primers pRSF-NcoI (ggt ata tct cct tat taa agt taa aca aaa tta ttt ctacag g) and pRSF-AvrII (taa cct agg ctg ctg cca ccg ctg agc aat aac) to homolog the DNA fragment shown in SEQ ID No.1 Recombined into the pRSFDuet-1 plasmid. After sequencing, it was shown that the small fragment between NcoI and AvrII of the restriction site of pRSFDu...
Embodiment 3
[0253] Example 3, the expression of alcohol dehydrogenase TbSADH gene or its mutants and the preparation of whole cells, crude enzyme solution and crude enzyme powder
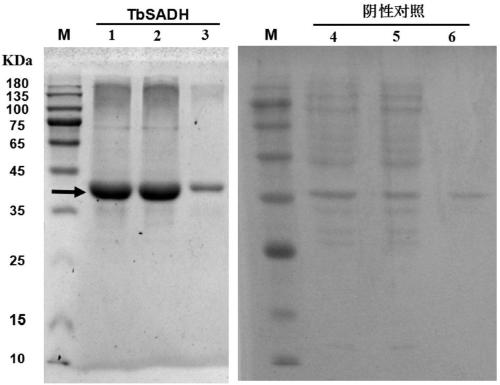
[0254] The alcohol dehydrogenase TbSADH gene wild-type genetic engineering strain prepared in step 1 of Example 2 and the 39 alcohol dehydrogenase TbSADH gene mutant engineering strains prepared in step 2 were induced and expressed to obtain wild-type alcohol dehydrogenase TbSADH and Individual alcohol dehydrogenase TbSADH mutants in Example 1. Specific steps are as follows:
[0255]Pick the recombinant E.coli BL21 (DE3) transformants containing the recombinant plasmid of the alcohol dehydrogenase TbSADH gene or its mutants respectively, and put them in 5 mL LB liquid medium containing 50 μg / mL kanamycin, 37 °C, 220 rpm Shake overnight for 12-16 hours, inoculate into TB liquid medium containing 50 μg / mL kanamycin according to 1% (volume percentage) inoculation amount, and culture at 37°C until OD 600 When the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com