Preparation and application of triphenylphosphine allyl palladium halide compound and derivative thereof
A technology of triphenylphosphine and compound, which is applied in the field of catalyst synthesis, can solve the problems of poor versatility of the method, high cost of materials, cumbersome process, etc., and achieve the effects of low cost, insensitivity to oxygen and heat, and fewer steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 3
[0103]Embodiment 1 Preparation of triphenylphosphine allyl palladium bromide and derivatives thereof
[0104] Step 1: Add 36mg of palladium acetate, 84mg of triphenylphosphine, 77mg of allyl bromide, 45mg of potassium tert-butoxide, and 1mL of 1,2-dichloroethane in a 50ml reaction tube, and heat to 80°C, react for 24 hours.
[0105] Step 2: After the reaction, cool to room temperature, dilute the reaction solution with dichloromethane (20 mL), filter, and dry under reduced pressure.
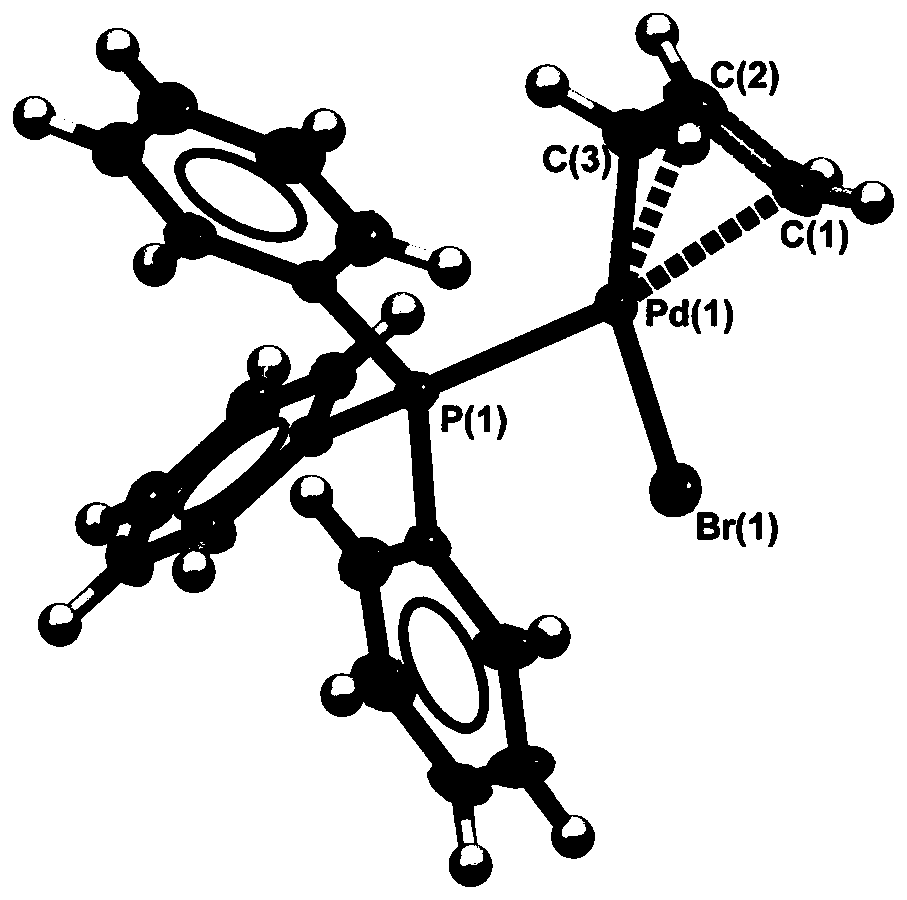
[0106] The third step: the crude product was stirred in petroleum ether / acetone (20:1 v / v) for 10 minutes, and filtered to obtain the product (product I) whose color and shape were yellow solids, and the yield could reach 85%. The yellow solid was recrystallized with dichloromethane / ether to obtain the structural formula [Pd(PPh 3 )(η 3 -C 3 h 5 ) Br] yellow crystal product, denoted as sample 1.
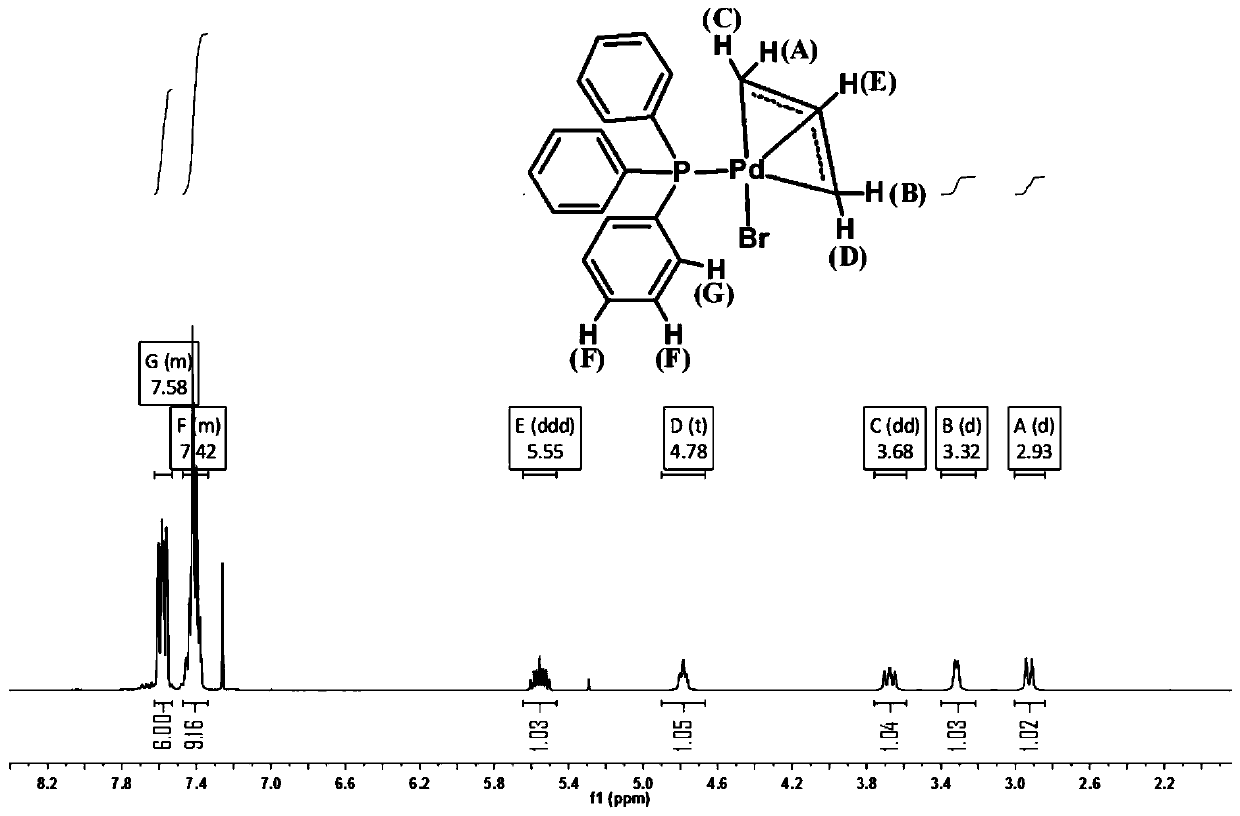
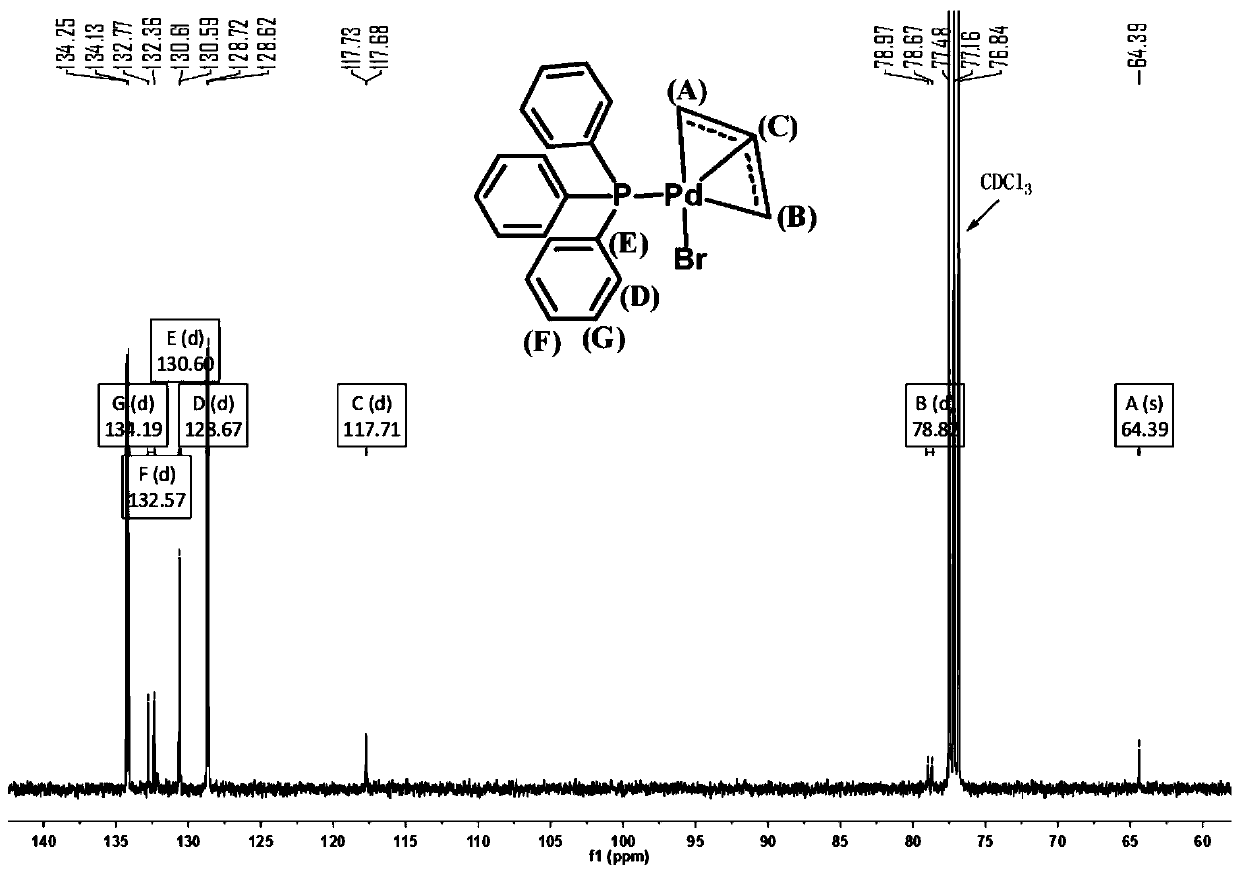
[0107] The product is carried out single crystal diffraction analysis, nuclear magnetic resona...
Embodiment 2 3
[0111] Embodiment 2 Preparation of three (p-methylphenyl) phosphine allyl palladium bromide and its derivatives
[0112] Step 1: Add 36mg of palladium acetate, 97mg of tris(p-methylphenyl)phosphine, 77mg of allyl bromide, 45mg of potassium tert-butoxide, 1mL of 1,2 dichloroethane in the reaction tube, under nitrogen atmosphere, Heated to 80°C for 24h.
[0113] Remaining steps are identical with embodiment 1. A divalent palladium yellow solid (product II) was obtained with a yield of up to 80%. The yellow solid was recrystallized from dichloromethane / ether to give the structure {Pd[P(p-Me-Ph) 3 ](η 3 -C 3 h 5 ) Br} yellow crystal product, denoted as sample 2.
[0114] The product is carried out single crystal diffraction analysis, nuclear magnetic resonance spectrum analysis and characterization, the results can be found in Figure 4 , Figure 5 and Figure 6 . Figure 4 It is a schematic diagram of the crystal structure of sample 2. It can be seen from the figure tha...
Embodiment 3 3
[0118] Example 3 Preparation of three (p-fluorophenyl) phosphine allyl palladium bromide and derivatives thereof
[0119] Step 1: Add 36mg of palladium acetate, 101mg of tris(p-fluorophenyl)phosphine, 77mg of allyl bromide, 45mg of potassium tert-butoxide, 1mL of 1,2 dichloroethane in a reaction tube, and heat under nitrogen atmosphere To 80°C, react for 24h.
[0120] Remaining steps are identical with embodiment 1. A divalent palladium yellow solid (product III) was obtained with a yield of up to 85%. The yellow solid was recrystallized from dichloromethane / ether to give the structure {Pd[P(p-F-Ph) 3 ](η 3 -C 3 h 5 ) Br} yellow crystal product, recorded as sample 3.
[0121] The product is carried out single crystal diffraction analysis, nuclear magnetic resonance spectrum analysis and characterization, the results can be found in Figure 7 , Figure 8 and Figure 9 . Figure 7 It is a schematic diagram of the crystal structure of sample 3. It can be seen from the f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com