Process method for preparing sodium 2-ketobutyrate
A process method and technology of sodium butanone, applied in the field of preparing 2-butanone sodium salt, can solve the problems of difficulty in scaling up to kilograms and ten kilograms, expensive Grignard reagents, harsh reaction conditions, etc., and avoid dangerous and expensive Reagents, to meet large-scale industrial production, the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
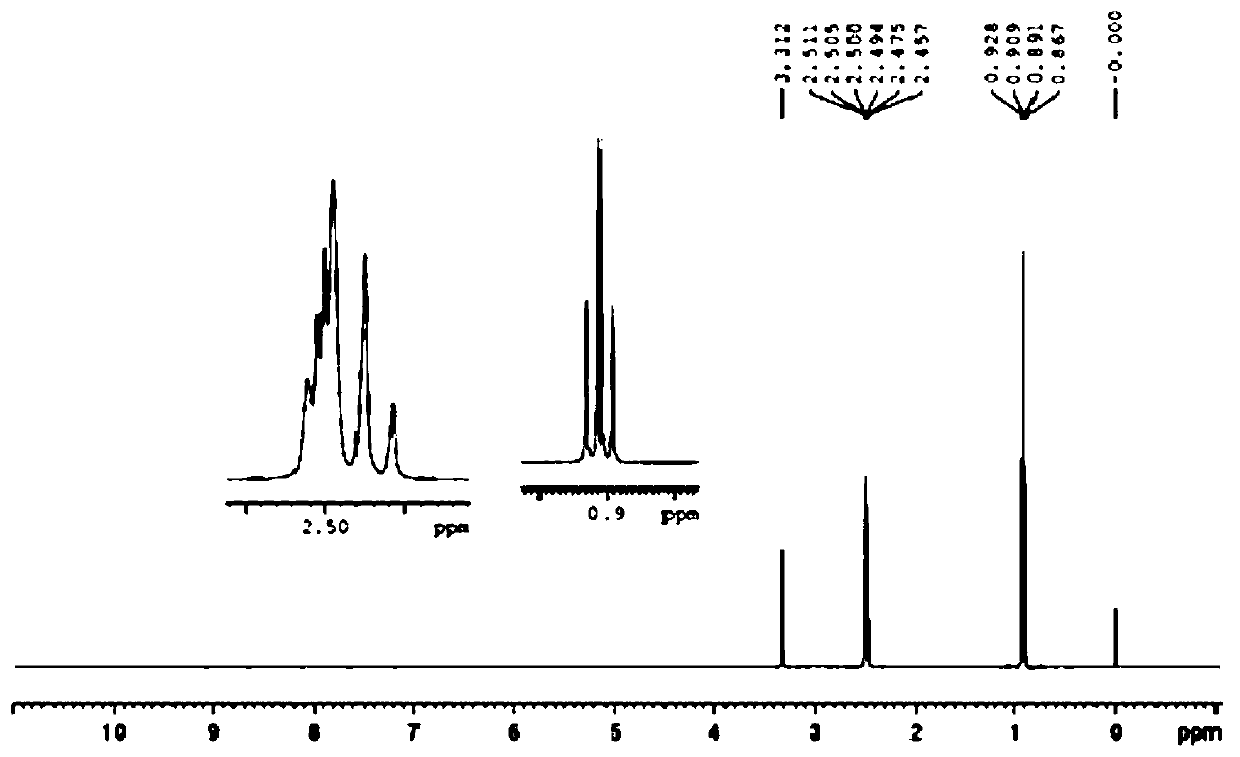
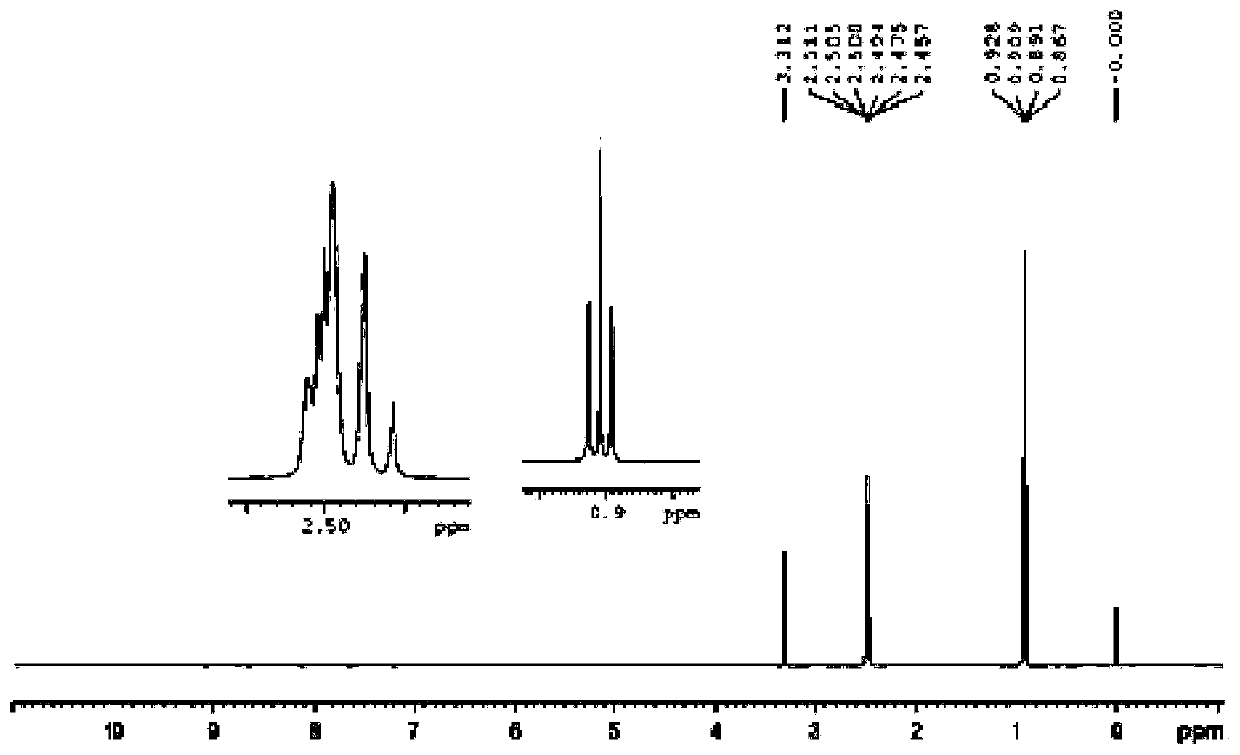
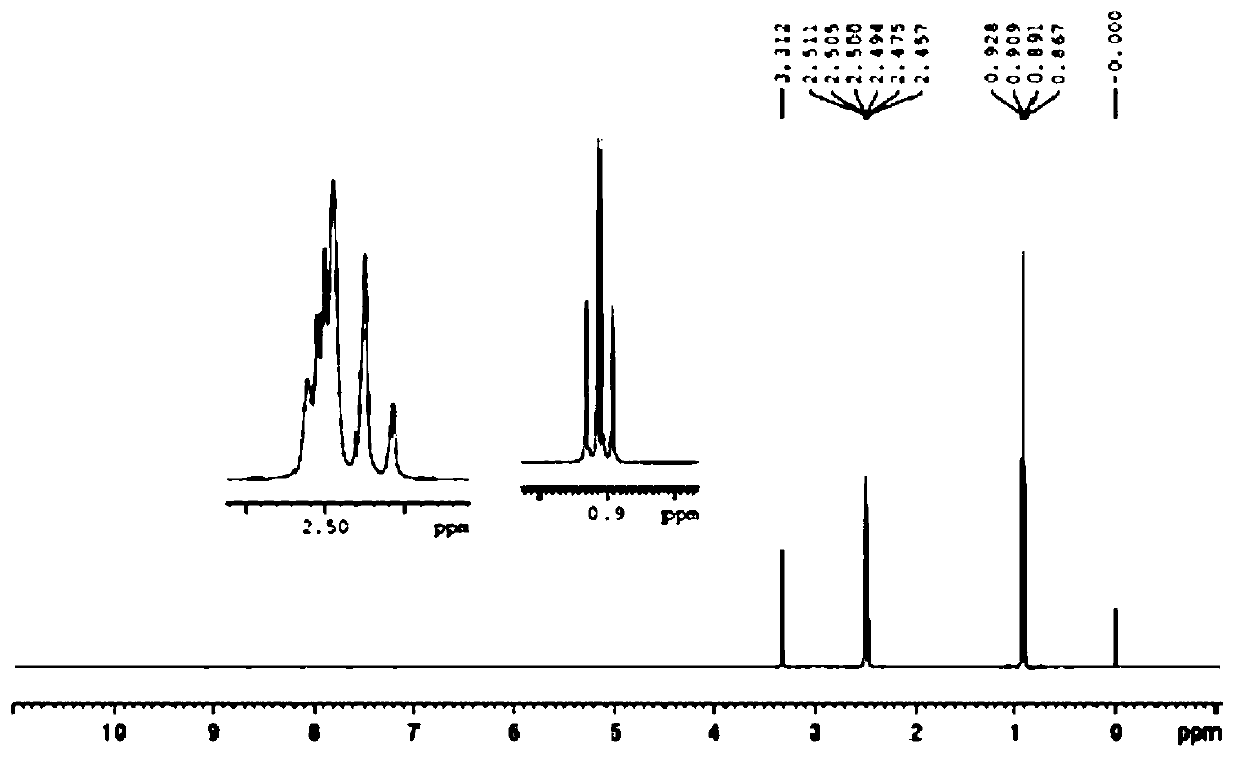
Image
Examples
Embodiment 1
[0044]
[0045] S1: Preparation of 1-carbonylpropane-1,2,2-tricarboxylic acid triethyl ester intermediate
[0046]
[0047] Under stirring at room temperature, 25 L of tert-butyl methyl ether, 8.7 Kg of diethyl methylmalonate and 7.5 Kg of diethyl oxalate were successively added into a 50 L glass reactor, and stirred evenly.
[0048]Further, 0.4Kg of sodium ethoxide was added in batches, and after the addition, the reaction solution was stirred at room temperature for 6 hours, and GC (gas chromatography) confirmed that the reaction was complete.
[0049] Further, hydrogen chloride ethanol solution was added dropwise to the reaction solution to adjust the pH of the reaction solution to 7.
[0050] Further, the reaction solution was first distilled under normal pressure to recover the solvent, and then distilled under reduced pressure to collect 11.8 Kg of 1-carbonylpropane-1,2,2-tricarboxylate triethyl intermediate in the form of yellow liquid, with a yield of 86%.
[00...
Embodiment 2
[0068]
[0069] S1: Preparation of 1-carbonylpropane-1,2,2-tricarboxylic acid trimethyl ester intermediate
[0070]
[0071] Under stirring at room temperature, 25L of tetrahydrofuran, 4.4Kg of dimethyl methylmalonate and 7.1Kg of dimethyl oxalate were successively added into a 50L glass reactor, and stirred evenly.
[0072] Further, 0.3Kg of sodium methoxide was added in batches. After the addition, the reaction solution was heated to 50° C., kept stirring for 5 hours, and GC (gas chromatography) confirmed that the reaction was complete.
[0073] Further, methanolic hydrogen chloride solution was added dropwise to the reaction solution to adjust the pH of the reaction solution to 7.
[0074] Further, the reaction liquid is firstly recovered by normal pressure distillation to recover the solvent, then the excess dimethyl oxalate is recovered by vacuum distillation, and then 6.4Kg of yellow liquid 1-carbonylpropane-1,2,2-tricarboxylic acid tricarboxylate is collected by v...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com