Method for resolving 3-chloro-phenylglycine enantiomers through liquid-liquid extraction
A technology for phenylglycine and enantiomers, which is applied in the field of liquid-liquid extraction and separation of 3-chloro-phenylglycine enantiomers, can solve the problems of low extraction efficiency, low distribution coefficient and the like, and achieves high extraction efficiency, high separation factor, Simple and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
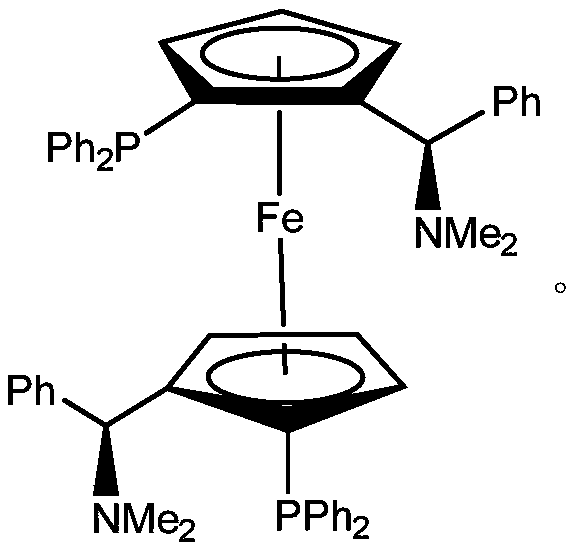
[0024] (1) Add 0.1mmol (S,S)-(-)-2,2'-bis[(R)-(N,N-dimethylamino)(phenyl)methyl]-1,1'- Dissolve bis(diphenylphosphino)ferrocene and 0.1mmol bis(acetonitrile)dichloropalladium in 100mL 1,2-dichloroethane, stir for 16 hours to form a chiral extraction with a concentration of 1.0mmol / L agent;
[0025] (2) Dissolve 0.2 mmol of 3-chloro-phenylglycine enantiomer in 100 mL of sodium dihydrogen phosphate / disodium hydrogen phosphate buffered aqueous solution with a pH value of 7.0 to obtain 2.0 mmol / L of 3-chloro-benzene Glycine enantiomer aqueous phase;
[0026] (3) Mix 10mL of the organic phase and the aqueous phase in step (1) and step (2), shake in a water bath constant temperature oscillator at 5°C for 24 hours, then let stand for 48 hours to separate the two phases, and apply high-efficiency liquid Determination of partition coefficient k of D-3-chloro-phenylglycine and L-3-chloro-phenylglycine and 3-chloro-phenylglycine in organic phase and aqueous phase respectively by phase ...
Embodiment 2
[0028] (1) Add 0.3mmol (S,S)-(-)-2,2'-bis[(R)-(N,N-dimethylamino)(phenyl)methyl]-1,1'- Bis(diphenylphosphino)ferrocene and 0.3mmol bis(acetonitrile)dichloropalladium were dissolved in 100mL of dichloromethane, stirred for 16 hours to form a chiral extractant with a concentration of 3.0mmol / L;
[0029] (2) Dissolve 0.2 mmol of 3-chloro-phenylglycine enantiomer in 100 mL of sodium dihydrogen phosphate / disodium hydrogen phosphate buffered aqueous solution with a pH value of 7.0 to obtain 2.0 mmol / L of 3-chloro-benzene Glycine enantiomer aqueous phase;
[0030] (3) Mix 10mL of the organic phase and the aqueous phase in step (1) and step (2), shake in a water bath constant temperature oscillator at 5°C for 24 hours, then let stand for 48 hours to separate the two phases, and apply high-efficiency liquid Determination of partition coefficient k of D-3-chloro-phenylglycine and L-3-chloro-phenylglycine and 3-chloro-phenylglycine in organic phase and aqueous phase respectively by phas...
Embodiment 3
[0032] (1) Add 0.1mmol (S,S)-(-)-2,2'-bis[(R)-(N,N-dimethylamino)(phenyl)methyl]-1,1'- Dissolve bis(diphenylphosphino)ferrocene and 0.1mmol palladium acetate in 100mL dichloromethane, stir for 17 hours to form a chiral extractant with a concentration of 1.0mmol / L;
[0033] (2) Dissolve 0.2 mmol of 3-chloro-phenylglycine enantiomer in 100 mL of sodium dihydrogen phosphate / disodium hydrogen phosphate buffered aqueous solution with a pH value of 7.0 to obtain 2.0 mmol / L of 3-chloro-benzene Glycine enantiomer aqueous phase;
[0034] (3) Mix 10mL of the organic phase and the aqueous phase in step (1) and step (2), shake in a water bath constant temperature oscillator at 10°C for 24 hours, then let stand for 48 hours to separate the two phases, and apply high-efficiency liquid Determination of partition coefficient k of D-3-chloro-phenylglycine and L-3-chloro-phenylglycine and 3-chloro-phenylglycine in organic phase and aqueous phase respectively by phase chromatography D and k L...
PUM
| Property | Measurement | Unit |
|---|---|---|
| separation factor | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com