Application of lncRNA to preparation of products for diagnosing and/or treating osteoarthritis
A technology for osteoarthritis and products, applied in the field of biomedicine, can solve the problems of limited treatment methods, treatment methods are limited to patients with advanced osteoarthritis, and late detection of osteoarthritis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
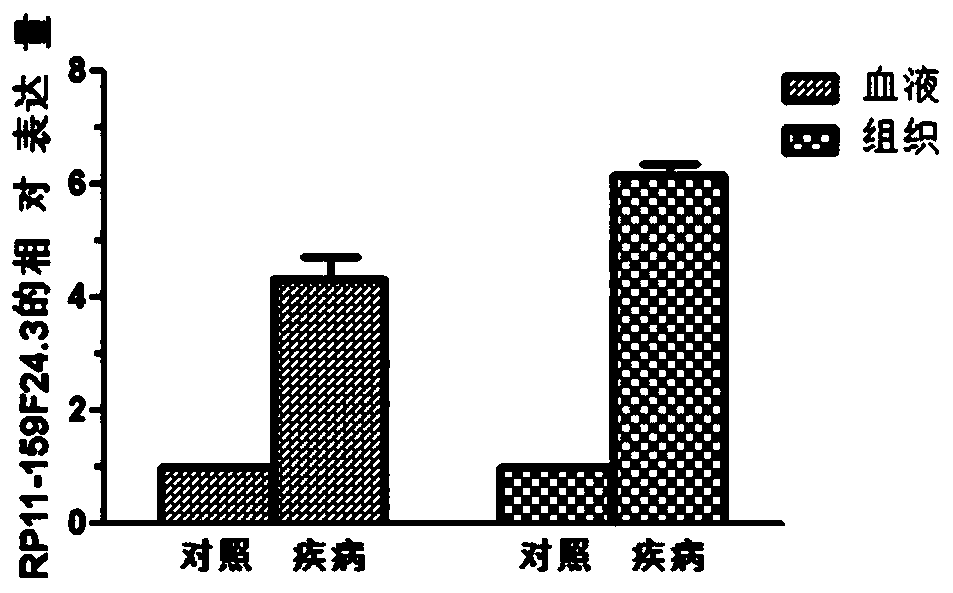
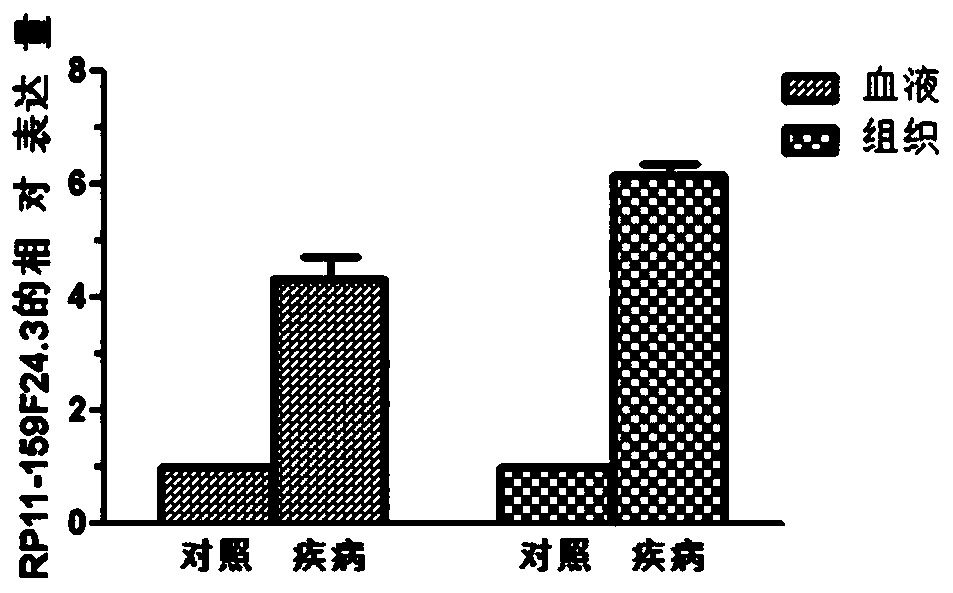
[0063] Example 1 QPCR sequencing to verify the expression of RP11-159F24.3 gene
[0064] 1. Sample collection
[0065] A total of 78 patients undergoing knee replacement surgery were selected, including 48 OA patients and 30 normal controls. Blood samples and tissue samples were collected from the patients. The age and gender of the OA group and the control group were not statistically significant.
[0066] OA group: Inclusion criteria: 1) Osteoarthritis patients diagnosed according to OA diagnostic criteria, underwent knee replacement surgery; 2) Initial replacement. Exclusion criteria: 1) Patients with other inflammatory arthritis or autoimmune diseases, including rheumatoid arthritis, gout, systemic lupus erythematosus, etc.; 2) Patients with a history of severe trauma to the knee joint; 3) Patients with steroids within the past 3 months Those with a history of drug injection or application of non-steroidal drugs; 4) those with severe liver and kidney function diseases and...
Embodiment 2
[0077] Example 2 Silencing of RP11-159F24.3 gene and its influence on cells
[0078] 1. Cell acquisition and culture
[0079] Repeatedly wash the bone tissue of soft OA patients with PBS containing 1% double antibody for 3 times, and cut the tissue to 1mm with sterile small ophthalmic scissors 3 Put the cartilage fragments into 10cm Petri dish, add 10ml of fresh 0.25% trypsin, 37℃, 5%CO 2 Digest in the incubator for 30min to remove possible attached fibroblasts. Aspirate the trypsin digestion solution, add 10ml of DMEM culture solution containing 10% FBS, pipette and mix well to stop the digestion, wash twice with 10ml of PBS containing double antibody. Add 10ml of 0.2% type II collagenase to the petri dish, place at 37°C, 5% CO 2 Digest for 4 hours in the incubator. The dissociated chondrocyte suspension containing collagenase solution was sucked out with a pipette, filtered through a 70 μm 200-mesh cell sieve, the obtained filtrate was transferred to a centrifuge tube an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com