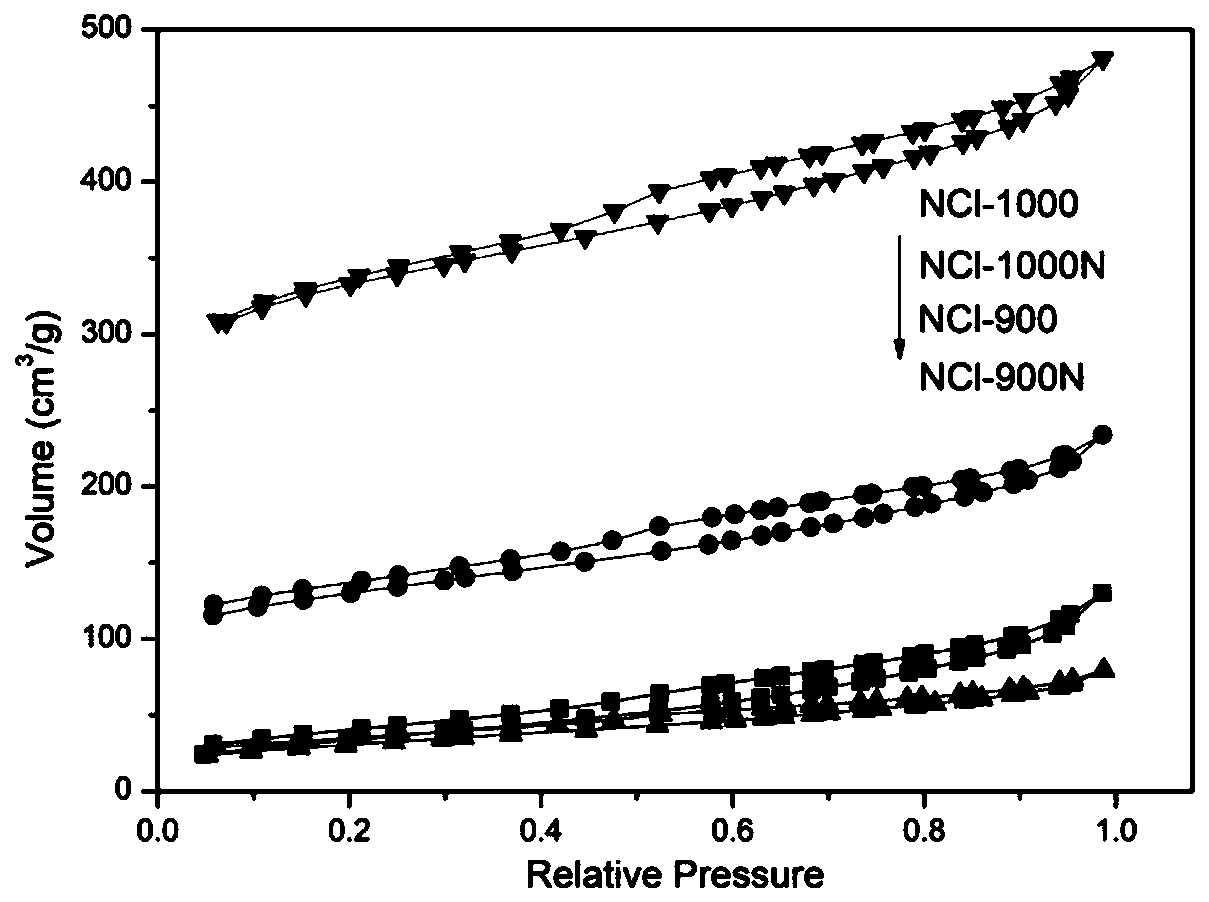
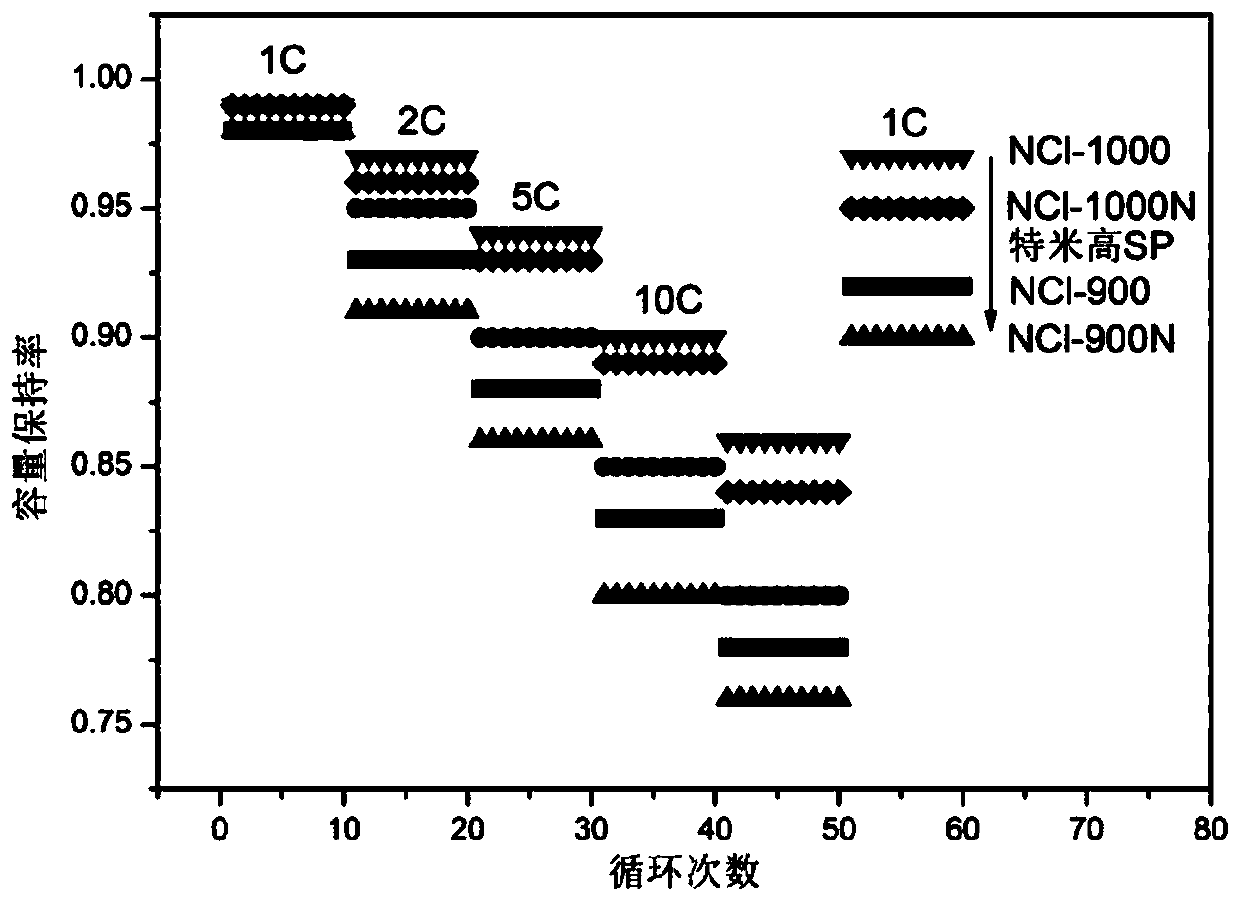
Preparation methods of nitrogen-chlorine co-doped polymer intermediate and nitrogen-chlorine co-doped polymer, nitrogen-chlorine co-doped polymer, and application of nitrogen-chlorine co-doped polymer
A co-doping and polymer technology, which is applied in the preparation/purification of carbon, electrical components, battery electrodes, etc., can solve the problems of adding a large amount of conductive agent, affecting the energy density of lithium-ion batteries, and reducing the amount of conductive agent added. , to achieve the effect of less input of raw materials, improved energy density and cycle performance, and improved utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: The reactant of nitrogen-chloride co-doped polymer
[0036] Accurately weigh 10g of aluminum chloride and add it to a 500mL glass reactor. Weigh 100g of hexachlorocyclopentadiene and 100g of acrylonitrile respectively, slowly add them to the stainless steel reactor, and heat the oil bath to 120°C for 1 hour. After the reaction was completed, it was naturally cooled to room temperature, and the resulting reactant was collected.
Embodiment 2
[0037] Embodiment 2: The reactant of nitrogen-chloride co-doped polymer
[0038] Accurately weigh 10g of aluminum chloride, add it into a 500mL glass reactor, weigh 10g of pentachlorocyclopentadiene and 100g of acrylonitrile, and slowly add them into a stainless steel reactor, heat the oil bath to 120°C for 1 hour. After the reaction was completed, it was naturally cooled to room temperature, and the resulting reactant was collected.
Embodiment 3
[0039] Embodiment 3: The reactant of nitrogen-chloride co-doped polymer
[0040] Accurately weigh 10g of aluminum chloride, add it to a 500mL glass reaction kettle, weigh 100g of hexachlorocyclopentadiene and 10g of crotononitrile, and slowly add them to the stainless steel reaction kettle, heat the oil bath to 120°C and react for 1h . After the reaction was completed, it was naturally cooled to room temperature, and the resulting reactant was collected.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com