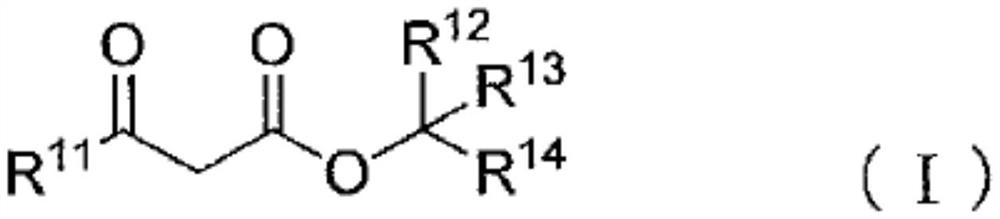
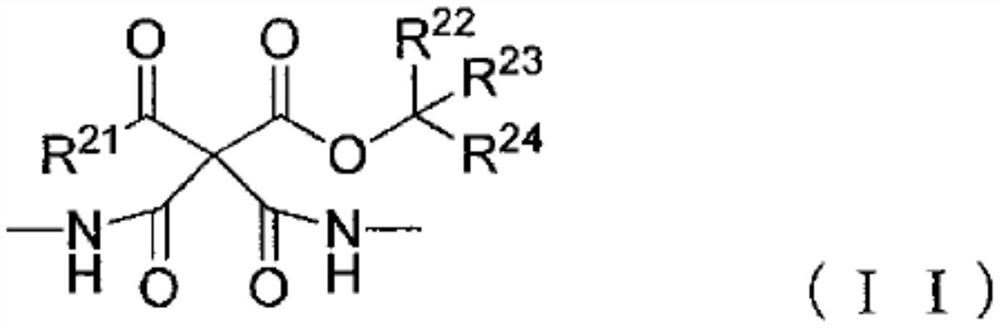
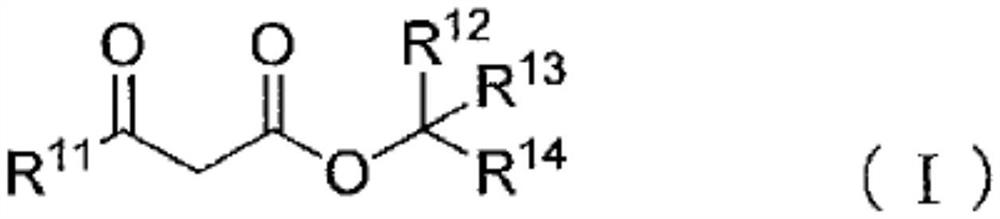
Blocked polyisocyanate composition and its application
A technology of polyisocyanate and diisocyanate, applied in the direction of polyurea/polyurethane adhesive, adhesive type, polyurea/polyurethane coating, etc., can solve the problem of shortening the heat treatment process of the easy-bonding treatment layer and failing to find the initial adhesion , Adhesion and other problems after the heat and humidity resistance test, to achieve the effects of excellent adhesion, excellent viscosity stability, hardness retention and excellent water resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1~1-13 and comparative example 1-1
[0635]
[0636] For the polyisocyanates obtained in the synthesis examples, the blocked polyisocyanate compositions produced in the examples and the comparative examples, the coating compositions containing the blocked polyisocyanate compositions, and the coating films obtained from the coating compositions, according to the following The measurement of each physical property and each evaluation were performed by the method shown.
[0637] [Property 1-1] Isocyanate group (NCO) content of polyisocyanate
[0638]Precisely weigh 1 to 3 g (Wg) of polyisocyanate in an Erlenmeyer flask. Next, 20 mL of toluene was added to dissolve the polyisocyanate. Next, 10 mL of a 2N toluene solution of di-n-butylamine was added, and after mixing, it was left at room temperature for 15 minutes. Next, 70 mL of isopropanol was added and mixed. Next, this solution was titrated with an indicator using 1N hydrochloric acid solution (Factor F). The resulting titer was V2mL. Next, the same operat...
Synthetic example 1-1
[0740] [Synthesis Example 1-1] Synthesis of Polyisocyanate P1-1
[0741] To a four-necked flask equipped with a thermometer, a stirring blade and a reflux cooling pipe, HDI: 1000 parts and trimethylolpropane: 33 g were charged under nitrogen flow, and the temperature in the reactor was maintained at 70°C while stirring. To this, tetramethylammonium hydroxide was added, and phosphoric acid was added to stop the reaction when the yield was 48%. After filtering the reaction liquid, unreacted HDI was removed by a thin-film distillation apparatus to obtain an isocyanurate-type polyisocyanate (hereinafter, sometimes referred to as "polyisocyanate P1-1").
[0742] The NCO content of the obtained polyisocyanate P1-1 was 19.9 mass %, the number average molecular weight was 1080, and the average number of isocyanate groups was 5.1.
Synthetic example 1-2
[0743] [Synthesis Example 1-2] Synthesis of Polyisocyanate P1-2
[0744] To a four-necked flask equipped with a thermometer, a stirring blade, and a reflux cooling pipe, HDI: 800 parts, IPDI: 200 g, and trimethylolpropane: 75 g were added under nitrogen flow, and the temperature in the reactor was kept at 70 while stirring. °C. To this, tetramethylammonium hydroxide was added, and phosphoric acid was added to stop the reaction when the yield was 46%. After filtering the reaction liquid, unreacted HDI and IPDI were removed by a thin-film distillation apparatus to obtain an isocyanurate-type polyisocyanate (hereinafter, sometimes referred to as "polyisocyanate P1-2").
[0745] The NCO content of the obtained polyisocyanate P1-2 was 18.5 mass %, the number average molecular weight was 1200, and the average number of isocyanate groups was 5.3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hydroxyl value | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com