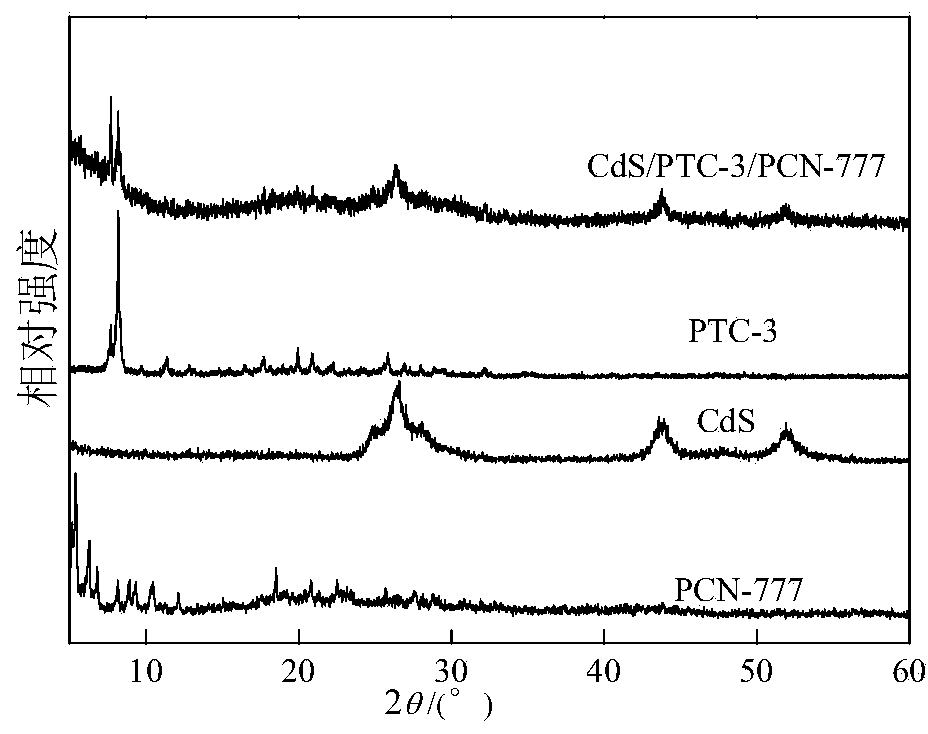
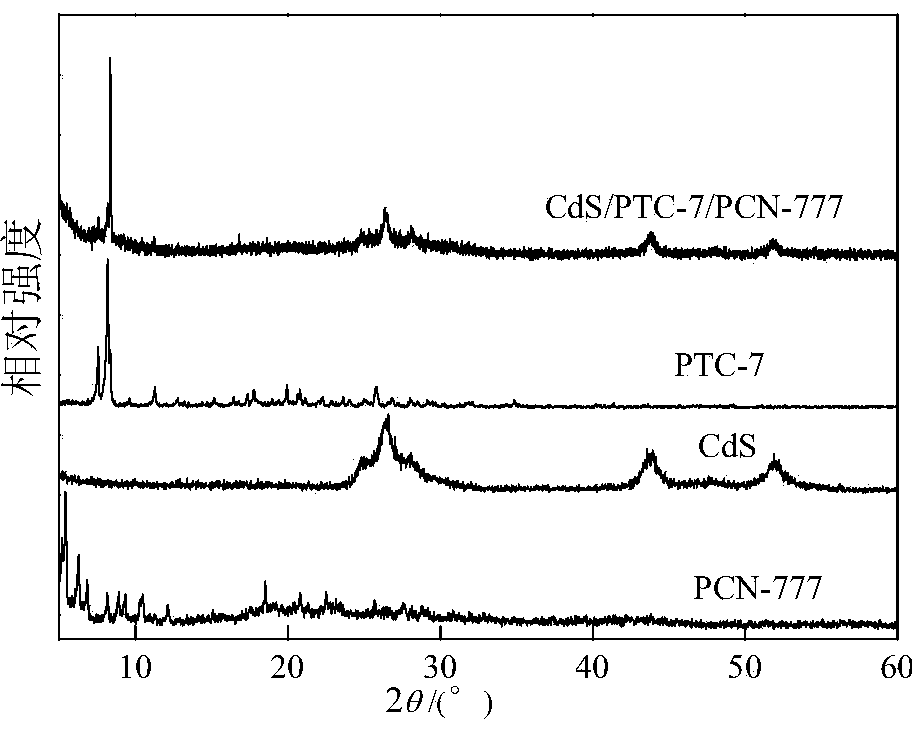
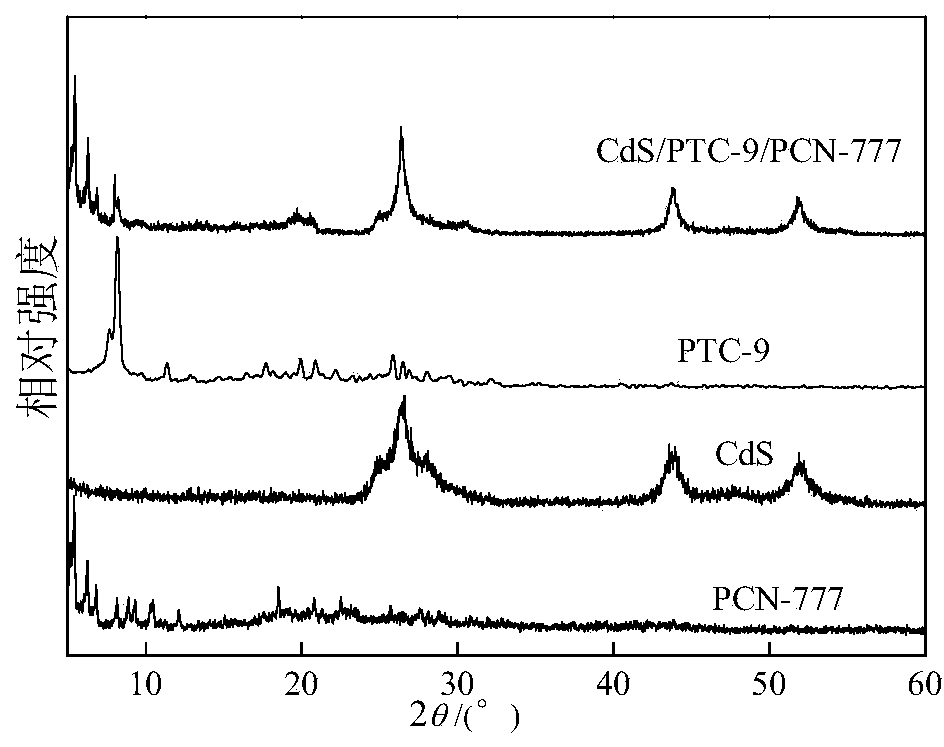
Multi-component composite titanium oxygen cluster (PTCs) CdSPCN-777 photocatalyst for decomposing water to produce hydrogen
A kind of PCN-777, photocatalyst technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] Specifically, a multi-component composite titanyl cluster (PTCs) CdSPCN-777 decomposes water and produces hydrogen photocatalyst, which is prepared by the following method:
[0030] A. Mix zirconium oxychloride, 2,4,6-tris(4-carboxyphenyl)-1,3,5-triazine, N,N-diethylformamide and trifluoroacetic acid evenly, React at 100-120°C, after the reaction is completed, after cooling, separation, washing and drying, the white solid product PCN-777 is obtained;
[0031] B. Add PCN-777 obtained in step A into water, then add cadmium salt, after dispersion, centrifuge filter; add the centrifuge filtered solid into water again, then add cadmium salt again, after dispersion, centrifuge again to obtain Cd-containing 2+ PCN-777;
[0032] C, with step B gained containing Cd 2+ Add PCN-777 into the autoclave, add water and sulfur source, react at 100-150 ° C, after the reaction is completed, after cooling, separation, washing and drying, the yellow CdS / PCN-777 photocatalyst is obtained;...
Embodiment 1
[0046] step one
[0047] Weigh 0.36g of zirconium oxychloride and 0.09g of 2,4,6-tris(4-carboxyphenyl)-1,3,5-triazine (TATB) into a 20mL reaction flask, then pipette 6mL N,N-diethylformamide (DEF) and 0.9mL of trifluoroacetic acid were added to a 20mL small glass bottle, placed on a magnetic stirrer and stirred for 30 minutes, then reacted in an oven at 120°C for 24 hours, and took out Naturally cooled to room temperature, centrifuged, washed several times with ethanol, and dried to obtain the white solid product PCN-777.
[0048] step two
[0049] Weigh 0.5g of the PCN-777 sample, add it to a beaker, add 25mL of deionized water to the beaker, then add 0.2g of cadmium acetate, stir at 40°C for 24 hours, then centrifugally filter; In the beaker, add 25mL deionized water and 0.2g cadmium acetate into the beaker, stir at 50°C for 12 hours, then add the solid sample obtained after centrifugal filtration into a 50mL autoclave liner, and add 25mL deionized water and Put 0.2g of t...
Embodiment 2
[0053] step one
[0054] Weigh 0.36g of zirconium oxychloride and 0.09g of 2,4,6-tris(4-carboxyphenyl)-1,3,5-triazine (TATB) into a 20mL reaction flask, then pipette 6mL Add N,N-diethylformamide (DEF) and 0.9mL trifluoroacetic acid into a 20mL small glass bottle, place it on a magnetic stirrer and stir for 30 minutes, react in an oven at 120°C for 24 hours, take out Naturally cooled to room temperature, centrifuged, washed several times with ethanol, and dried to obtain the white solid product PCN-777.
[0055] step two
[0056] Weigh 1.0g of PCN-777 sample, add it to a beaker, add 25mL deionized water to the beaker, then add 0.5g cadmium acetate, stir at 40°C for 24 hours, and then centrifugally filter; add the solid sample obtained by centrifugal filtration Add 25mL of deionized water and 0.5g of cadmium acetate into the beaker, stir at 50°C for 12 hours, then add the solid sample obtained after centrifugal filtration into a 50mL autoclave liner, and then add 25mL of deion...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com