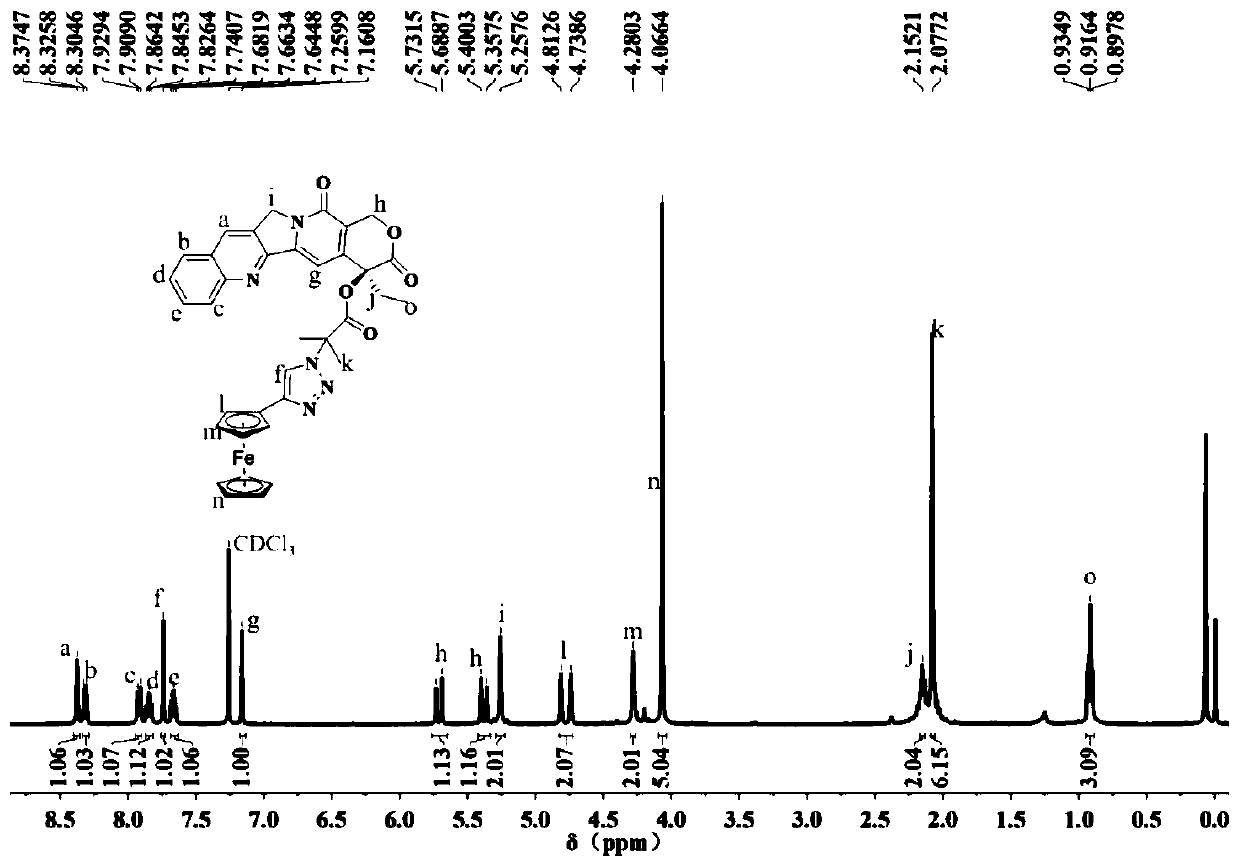
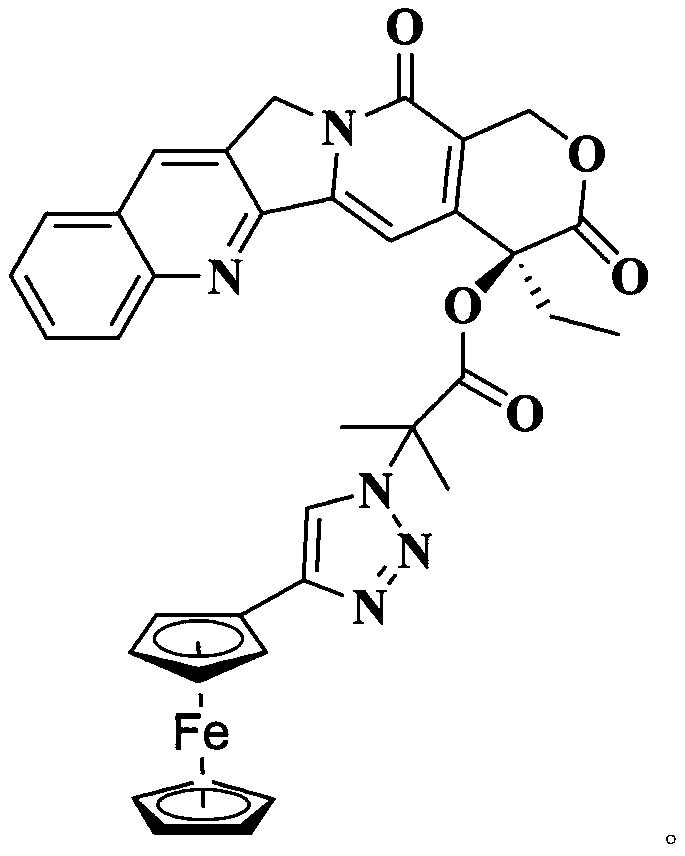
Organic metal drug camptothecin-ferrocene and preparation method thereof
An organometallic and camptothecin technology, applied in the field of organometallic drug camptothecin-ferrocene and its preparation, can solve the problems of cumbersome preparation methods and low yield of derivatives, and achieve the effect of simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1. Under ice-bath conditions, add 50 mg camptothecin to a 100 ml dry single-necked flask, add 10 ml of dichloromethane to dissolve, then add 80 μl of triethylamine and keep stirring. Measure 80 μl of bromoisobutyryl bromide and dissolve it in 10ml of dichloromethane, slowly drop it into the ice-bath reaction system, stir for 30 minutes after the dropwise addition, transfer to room temperature for stirring, and react for 12 hours. After the reaction was finished, extract twice with saturated sodium bicarbonate solution, remove the lower organic phase, concentrate the liquid by rotary evaporation, pass through a silica gel column, get the filtrate, and obtain 68 mg of white bromocamptothecin solid after evaporating the solvent with a rotary evaporator.
[0031] 2. Add 68mg of bromocamptothecin, 20mg of sodium azide and 45mg of cesium chloride into a dry 100ml single-necked flask, add 10ml of N,N-dimethylformamide as a solvent, and heat at 60°C Stirring, reaction 12h. Aft...
Embodiment 2
[0035] 1. Under ice-bath conditions, add 100 mg of camptothecin to a 100 ml dry single-necked flask, add 15 ml of dichloromethane to dissolve, then add 160 μl of triethylamine and keep stirring. Measure 150 μl of bromoisobutyryl bromide and dissolve it in 10ml of dichloromethane, slowly drop it into the ice-bath reaction system, stir for 30 minutes after the dropwise addition, transfer to room temperature and stir, and react for 12 hours. After completion of the reaction, extract twice with saturated sodium bicarbonate solution, remove the lower organic phase, concentrate the liquid by rotary evaporation, pass through a silica gel column, get the filtrate, and obtain 156 mg of bromocamptothecin solids after evaporating the solvent to dryness with a rotary evaporator.
[0036] 2. Add 156mg of bromocamptothecin, 35mg of sodium azide and 86mg of cesium chloride to a dry 100ml single-necked flask, add 15ml of N,N-dimethylformamide as a solvent, and set the temperature at 60°C Heat...
Embodiment 3
[0039] 1. Under ice-bath conditions, add 200 mg camptothecin to a 100 ml dry single-necked flask, add 20 ml of dichloromethane to dissolve, then add 350 μl of triethylamine and keep stirring. Measure 300 μl of bromoisobutyryl bromide and dissolve it in 15ml of dichloromethane, slowly drop it into the ice-bath reaction system, stir for 30 minutes after the dropwise addition, transfer to room temperature and stir, and react for 12 hours. After completion of the reaction, extract twice with saturated sodium bicarbonate solution, take the lower organic phase, concentrate the liquid by rotary evaporation, pass through a silica gel column, get the filtrate, and obtain 302mg of bromocamptothecin solids after evaporating the solvent to dryness with a rotary evaporator.
[0040] 2. Add 302mg of tribromogemcitabine, 70mg of sodium azide and 170mg of cesium chloride into a dry 100ml single-necked flask, add 20ml of N,N-dimethylformamide as a solvent, and heat at 60°C Stirring, reaction 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com