Method for detecting soluble epoxide hydrolase by liquid chromatography-mass spectrometry
An epoxide and LC/MS technology, applied in measurement devices, instruments, scientific instruments, etc., can solve the problems of poor specificity, difficult to measure large quantities of samples, etc., and achieve the effect of high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
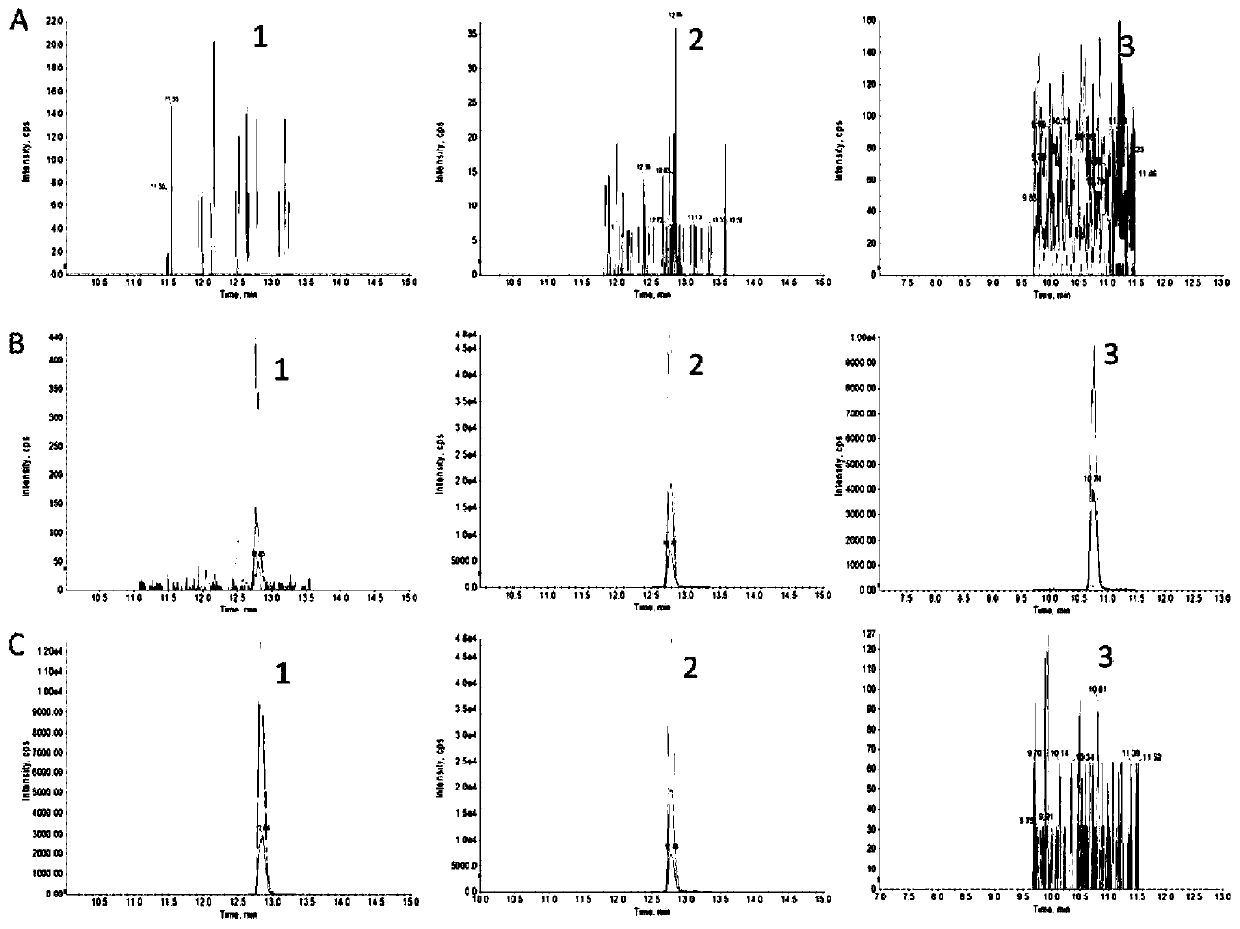
[0036] Example 1: Determination of the concentration of sEH protein in rat heart, liver and kidney tissues
[0037] 1. Experimental materials and instruments
[0038] Materials: DFLLGAFQMK standard peptides and EKDFLLGAFQMK peptidomimetics: Jiangsu GenScript Biotechnology Co., Ltd., content greater than 98.5%; DFLLGAFQMK* isotope peptide stock solution: Thermo Scientific, USA, content greater than 95%; BCA kit, DDT, IAA and Pancreatin: Thermo Scientific; Acetonitrile and methanol: Merck Company, HPLC grade; Formic acid: ACS Company, HPLC grade; Water was prepared by Millipore Milli-Q Advantage A10 ultrapure water machine.
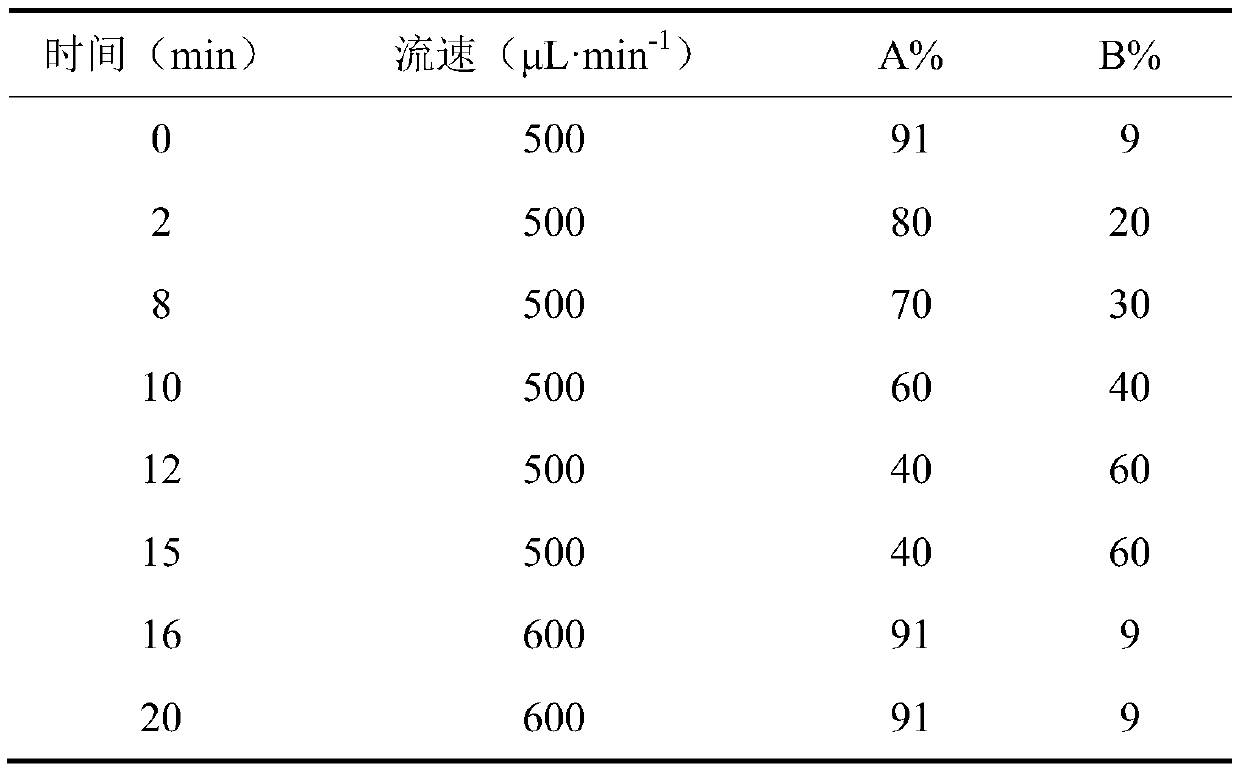
[0039] Instrument: API 4000 LC-MS / MS LC-MS, including: (1) Pump Agilent 1260G1312A; (2) Column Oven Agilent 1260G1316A; (3) Auto Sampler Agilent 1260G1367E; (4) Massspectrometer API4000; (5) Value Valco 2-Position, controlled by Analyst 1.6 Software; WH-2 Mini Vortex Mixer (Shanghai Huxi Analytical Instrument Factory); MS-100 Constant Temperature Mixer (Hangzhou ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| correlation coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com